Modulation of arachidonic and linoleic acid metabolites in myeloperoxidase-deficient mice during acute inflammation
- PMID: 20156554
- PMCID: PMC2856720
- DOI: 10.1016/j.freeradbiomed.2010.02.010
Modulation of arachidonic and linoleic acid metabolites in myeloperoxidase-deficient mice during acute inflammation
Abstract
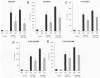
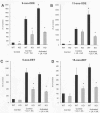
Acute inflammation is a common feature of many life-threatening pathologies, including septic shock. One hallmark of acute inflammation is the peroxidation of polyunsaturated fatty acids forming bioactive products that regulate inflammation. Myeloperoxidase (MPO) is an abundant phagocyte-derived hemoprotein released during phagocyte activation. Here, we investigated the role of MPO in modulating biologically active arachidonic acid (AA) and linoleic acid (LA) metabolites during acute inflammation. Wild-type and MPO-knockout (KO) mice were exposed to intraperitoneally injected endotoxin for 24 h, and plasma LA and AA oxidation products were comprehensively analyzed using a liquid chromatography-mass spectrometry method. Compared to wild-type mice, MPO-KO mice had significantly lower plasma levels of LA epoxides and corresponding LA- and AA-derived fatty acid diols. AA and LA hydroxy intermediates (hydroxyeicosatetraenoic and hydroxyoctadecadienoic acids) were also significantly lower in MPO-KO mice. Conversely, MPO-deficient mice had significantly higher plasma levels of cysteinyl-leukotrienes with well-known proinflammatory properties. In vitro experiments revealed significantly lower amounts of AA and LA epoxides, LA- and AA-derived fatty acid diols, and AA and LA hydroxy intermediates in stimulated polymorphonuclear neutrophils isolated from MPO-KO mice. Our results demonstrate that MPO modulates the balance of pro- and anti-inflammatory lipid mediators during acute inflammation and, in this way, may control acute inflammatory diseases.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Neither linoleic acid nor arachidonic acid promote white adipose tissue inflammation in Fads2-/- mice fed low fat diets.Prostaglandins Leukot Essent Fatty Acids. 2017 Nov;126:84-91. doi: 10.1016/j.plefa.2017.09.008. Epub 2017 Sep 15. Prostaglandins Leukot Essent Fatty Acids. 2017. PMID: 29031400
-
The effect of n-3 fatty acids and coenzyme Q10 supplementation on neutrophil leukotrienes, mediators of inflammation resolution and myeloperoxidase in chronic kidney disease.Prostaglandins Other Lipid Mediat. 2018 May;136:1-8. doi: 10.1016/j.prostaglandins.2018.03.002. Epub 2018 Mar 22. Prostaglandins Other Lipid Mediat. 2018. PMID: 29577973 Clinical Trial.
-
Obesity is positively associated with arachidonic acid-derived 5- and 11-hydroxyeicosatetraenoic acid (HETE).Metabolism. 2017 May;70:177-191. doi: 10.1016/j.metabol.2017.01.034. Epub 2017 Feb 7. Metabolism. 2017. PMID: 28403941
-
The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process.Int J Mol Sci. 2020 Dec 17;21(24):9628. doi: 10.3390/ijms21249628. Int J Mol Sci. 2020. PMID: 33348841 Free PMC article. Review.
-
Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: generation of antiinflammatory and antiproliferative metabolites.Am J Clin Nutr. 2000 Jan;71(1 Suppl):361S-6S. doi: 10.1093/ajcn/71.1.361s. Am J Clin Nutr. 2000. PMID: 10617998 Review.
Cited by
-
Pellino1 deficiency reprograms cardiomyocytes energy metabolism in lipopolysaccharide-induced myocardial dysfunction.Amino Acids. 2021 May;53(5):713-737. doi: 10.1007/s00726-021-02978-w. Epub 2021 Apr 22. Amino Acids. 2021. PMID: 33885999 Free PMC article.
-
Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their "Balanced Antagonistic Metabolic Functions" in the Human Body.J Lipids. 2021 Mar 17;2021:8848161. doi: 10.1155/2021/8848161. eCollection 2021. J Lipids. 2021. PMID: 33815845 Free PMC article. Review.
-
A real - life observational pilot study to evaluate the effects of two-week treatment with montelukast in patients with chronic cough.Cough. 2014 Mar 20;10(1):2. doi: 10.1186/1745-9974-10-2. Cough. 2014. PMID: 24649919 Free PMC article.
-
Identification and profiling of targeted oxidized linoleic acid metabolites in rat plasma by quadrupole time-of-flight mass spectrometry.Biomed Chromatogr. 2013 Apr;27(4):422-32. doi: 10.1002/bmc.2809. Epub 2012 Oct 5. Biomed Chromatogr. 2013. PMID: 23037960 Free PMC article.
-
Neutrophils in tissue injury and repair.Cell Tissue Res. 2018 Mar;371(3):531-539. doi: 10.1007/s00441-017-2785-7. Epub 2018 Jan 30. Cell Tissue Res. 2018. PMID: 29383445 Free PMC article. Review.
References
-
- Aldridge AJ. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg. 2002;168:204–214. - PubMed
-
- Winterbourn CC, Vissers MC, Kettle AJ. Myeloperoxidase. Curr Opin Hematol. 2000;7:53–58. - PubMed
-
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. - PubMed
-
- Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281:39860–39869. - PubMed
-
- Nauseef WM. Contributions of myeloperoxidase to proinflammatory events: more than an antimicrobial system. Int J Hematol. 2001;74:125–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

