Laminin chain assembly is regulated by specific coiled-coil interactions
- PMID: 20156561
- PMCID: PMC2877795
- DOI: 10.1016/j.jsb.2010.02.004
Laminin chain assembly is regulated by specific coiled-coil interactions
Abstract
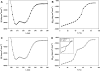
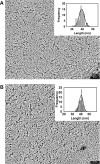
Laminins are large heterotrimeric, multidomain proteins that play a central role in organising and establishing all basement membranes. Despite a total of 45 potential heterotrimeric chain combinations formed through the coiled-coil domain of the 11 identified laminin chains (alpha1-5, beta1-3, gamma1-3), to date only 15 different laminin isoforms have been reported. This observation raises the question whether laminin assembly is regulated by differential gene expression or specific chain recognition. To address this issue, we here perform a complete analysis of laminin chain assembly and specificity. Using biochemical and biophysical techniques, all possible heterotrimeric combinations from recombinant C-terminal coiled-coil fragments of all chains were analysed. Apart from laminin 323 (alpha3, beta2, gamma3), for which no biochemical evidence of its existence in vivo is available, these experiments confirmed all other known laminin isoforms and identified two novel potential chain combinations, laminins 312 (alpha3, beta1, gamma2) and 422 (alpha4, beta2, gamma4). Our findings contribute to the understanding of basement membrane structure, function and diversity.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
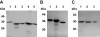


Similar articles
-
Laminin gamma3 chain binds to nidogen and is located in murine basement membranes.J Biol Chem. 2005 Jun 10;280(23):22146-53. doi: 10.1074/jbc.M501875200. Epub 2005 Apr 11. J Biol Chem. 2005. PMID: 15824114
-
The C-terminal region of laminin beta chains modulates the integrin binding affinities of laminins.J Biol Chem. 2009 Mar 20;284(12):7820-31. doi: 10.1074/jbc.M809332200. Epub 2009 Jan 15. J Biol Chem. 2009. PMID: 19147489 Free PMC article.
-
Interaction of agrin with laminin requires a coiled-coil conformation of the agrin-binding site within the laminin gamma1 chain.EMBO J. 1999 Dec 1;18(23):6762-70. doi: 10.1093/emboj/18.23.6762. EMBO J. 1999. PMID: 10581249 Free PMC article.
-
Laminin isoforms and lung development: all isoforms are not equal.Dev Biol. 2006 Jun 15;294(2):271-9. doi: 10.1016/j.ydbio.2006.03.032. Epub 2006 Apr 3. Dev Biol. 2006. PMID: 16643883 Review.
-
Functional sites in the laminin alpha chains.Connect Tissue Res. 2005;46(3):142-52. doi: 10.1080/03008200591008527. Connect Tissue Res. 2005. PMID: 16147852 Review.
Cited by
-
Laminins containing the β2 and γ3 chains regulate astrocyte migration and angiogenesis in the retina.Development. 2013 May;140(9):2050-60. doi: 10.1242/dev.087817. Development. 2013. PMID: 23571221 Free PMC article.
-
The laminin family: founding members of the basement membrane.Cell Adh Migr. 2013 Jan-Feb;7(1):44-7. doi: 10.4161/cam.23276. Epub 2012 Dec 21. Cell Adh Migr. 2013. PMID: 23263635 Free PMC article. No abstract available.
-
The laminin family.Cell Adh Migr. 2013 Jan-Feb;7(1):48-55. doi: 10.4161/cam.22826. Epub 2012 Dec 21. Cell Adh Migr. 2013. PMID: 23263632 Free PMC article. Review.
-
Development of Self-Assembled Nanoribbon Bound Peptide-Polyaniline Composite Scaffolds and Their Interactions with Neural Cortical Cells.Bioengineering (Basel). 2018 Jan 13;5(1):6. doi: 10.3390/bioengineering5010006. Bioengineering (Basel). 2018. PMID: 29342881 Free PMC article.
-
Novel Acellular Scaffold Made from Decellularized Schwann Cell Sheets for Peripheral Nerve Regeneration.Regen Eng Transl Med. 2015 Dec;1(1):22-31. doi: 10.1007/s40883-015-0003-2. Epub 2015 Oct 4. Regen Eng Transl Med. 2015. PMID: 26848489 Free PMC article.
References
-
- Deutzmann R., Huber J., Schmetz K.A., Oberbaumer I., Hartl L. Structural study of long arm fragments of laminin. Evidence for repetitive C-terminal sequences in the A-chain, not present in the B-chains. Eur. J. Biochem. 1988;177:35–45. - PubMed
-
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967;6:1948–1954. - PubMed
-
- Engel J., Odermatt E., Engel A., Madri J.A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J. Mol. Biol. 1981;150:97–120. - PubMed
-
- Fowler W.E., Aebi U. Preparation of single molecules and supramolecular complexes for high-resolution metal shadowing. J. Ultrastruct. Res. 1983;83:319–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

