Laminin chain assembly is regulated by specific coiled-coil interactions
- PMID: 20156561
- PMCID: PMC2877795
- DOI: 10.1016/j.jsb.2010.02.004
Laminin chain assembly is regulated by specific coiled-coil interactions
Abstract
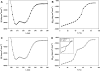
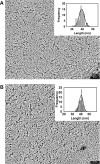
Laminins are large heterotrimeric, multidomain proteins that play a central role in organising and establishing all basement membranes. Despite a total of 45 potential heterotrimeric chain combinations formed through the coiled-coil domain of the 11 identified laminin chains (alpha1-5, beta1-3, gamma1-3), to date only 15 different laminin isoforms have been reported. This observation raises the question whether laminin assembly is regulated by differential gene expression or specific chain recognition. To address this issue, we here perform a complete analysis of laminin chain assembly and specificity. Using biochemical and biophysical techniques, all possible heterotrimeric combinations from recombinant C-terminal coiled-coil fragments of all chains were analysed. Apart from laminin 323 (alpha3, beta2, gamma3), for which no biochemical evidence of its existence in vivo is available, these experiments confirmed all other known laminin isoforms and identified two novel potential chain combinations, laminins 312 (alpha3, beta1, gamma2) and 422 (alpha4, beta2, gamma4). Our findings contribute to the understanding of basement membrane structure, function and diversity.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
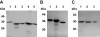


References
-
- Deutzmann R., Huber J., Schmetz K.A., Oberbaumer I., Hartl L. Structural study of long arm fragments of laminin. Evidence for repetitive C-terminal sequences in the A-chain, not present in the B-chains. Eur. J. Biochem. 1988;177:35–45. - PubMed
-
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967;6:1948–1954. - PubMed
-
- Engel J., Odermatt E., Engel A., Madri J.A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J. Mol. Biol. 1981;150:97–120. - PubMed
-
- Fowler W.E., Aebi U. Preparation of single molecules and supramolecular complexes for high-resolution metal shadowing. J. Ultrastruct. Res. 1983;83:319–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

