Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles
- PMID: 20156837
- PMCID: PMC2855360
- DOI: 10.1093/toxsci/kfq041
Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles
Abstract
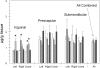
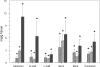
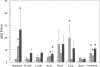
Titanium dioxide (TiO(2)) is included in some sunscreen formulations to physically block ultraviolet radiation. A dermal penetration study was conducted in minipigs with three TiO(2) particles (uncoated submicron sized, uncoated nano-sized, and dimethicone/methicone copolymer-coated nanosized) applied 5% by weight in a sunscreen. These and control formulations were topically applied to minipigs at 2 mg cream/cm(2) skin (4 applications/day, 5 days/week, 4 weeks). Skin (multiple sites), lymph nodes, liver, spleen, and kidneys were removed, and the TiO(2) content was determined (as titanium) using inductively coupled plasma mass spectroscopy. Titanium levels in lymph nodes and liver from treated animals were not increased over the values in control animals. The epidermis from minipigs treated with sunscreens containing TiO(2) showed elevated titanium. Increased titanium was detected in abdominal and neck dermis of minipigs treated with uncoated and coated nanoscale TiO(2). Using electron microscopy-energy dispersive x-ray analysis, all three types of TiO(2) particles were found in the stratum corneum and upper follicular lumens in all treated skin samples (more particles visible with coated nanoscale TiO(2)). Isolated titanium particles were also present at various locations in the dermis of animals treated with all three types of TiO(2)-containing sunscreens; however, there was no pattern of distribution or pathology suggesting the particles could be the result of contamination. At most, the few isolated particles represent a tiny fraction of the total amount of applied TiO(2). These findings indicate that there is no significant penetration of TiO(2) nanoparticles through the intact normal epidermis.
Figures
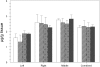




References
-
- Bennat C, Müller-Goymann CC. Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int. J. Cosmetic Sci. 2000;22:271–283. - PubMed
-
- Blake DM, Maness P-C, Huang Z, Wolfrum EJ, Jacoby WA, Huang J. Application of the photocatalytic chemistry of titanium dioxide to disinfection and the killing of cancer cells. Sep. Purif. Methods. 1999;28:1–50.
-
- Brand RM, McMahon L, Jendrzejewski JL, Charron AR. Transdermal absorption of the herbicide 2,4-dichlorophenoxyacetic acid is enhanced by both ethanol consumption and sunscreen application. Food Chem. Toxicol. 2007;45:93–97. - PubMed
-
- Cross SE, Innes B, Roberts MS, Tsuzuki T, Robertson TA, McCormick P. Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol. Physiol. 2007;20:148–154. - PubMed
-
- Dunford R, Salinaro A, Cai L, Serpone N, Horikoshi S, Hidaka H, Knowland J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418:87–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

