Perisomatic GABAergic innervation in prefrontal cortex is regulated by ankyrin interaction with the L1 cell adhesion molecule
- PMID: 20156840
- PMCID: PMC2981022
- DOI: 10.1093/cercor/bhq016
Perisomatic GABAergic innervation in prefrontal cortex is regulated by ankyrin interaction with the L1 cell adhesion molecule
Abstract
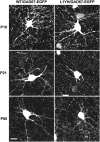
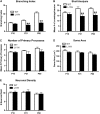
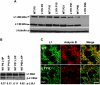
The L1 adhesion molecule functions in axon growth and guidance, but a role in synaptic development of cortical inhibitory interneurons is largely unexplored. L1 mediates adhesion by engaging the actin cytoskeleton through binding the actin/spectrin adapter protein ankyrin. Loss of L1-ankyrin interaction impaired process elaboration/branching by GABAergic interneurons, including basket cells, and reduced the number of perisomatic synapses in the cingulate cortex as shown in L1 mutant mice (L1YH) with a mutation in the ankyrin-binding site, either alone or intercrossed with GAD67-enhanced green fluorescence protein reporter mice. Electron microscopy revealed that perisomatic inhibitory synapses but not excitatory synapses in the neuropil were specifically affected. In wild-type cingulate cortex, L1 colocalized with perisomatic synaptic markers, whereas L1 phosphorylation on Tyr(1229) decreased postnatally, correlating with increased ankyrin binding and synaptic development. These results suggest a novel role for L1 engagement with the actin cytoskeleton in development of inhibitory connectivity within the cingulate cortex.
Figures




Similar articles
-
Polysialylated NCAM and ephrinA/EphA regulate synaptic development of GABAergic interneurons in prefrontal cortex.Cereb Cortex. 2013 Jan;23(1):162-77. doi: 10.1093/cercor/bhr392. Epub 2012 Jan 23. Cereb Cortex. 2013. PMID: 22275477 Free PMC article.
-
Reduced GABAergic transmission and number of hippocampal perisomatic inhibitory synapses in juvenile mice deficient in the neural cell adhesion molecule L1.Mol Cell Neurosci. 2004 May;26(1):191-203. doi: 10.1016/j.mcn.2004.01.008. Mol Cell Neurosci. 2004. PMID: 15121190
-
Developmental regulation of GABAergic interneuron branching and synaptic development in the prefrontal cortex by soluble neural cell adhesion molecule.Mol Cell Neurosci. 2008 Apr;37(4):781-93. doi: 10.1016/j.mcn.2008.01.006. Epub 2008 Jan 17. Mol Cell Neurosci. 2008. PMID: 18289872 Free PMC article.
-
Subcellular organization of GABAergic synapses: role of ankyrins and L1 cell adhesion molecules.Nat Neurosci. 2006 Feb;9(2):163-6. doi: 10.1038/nn1638. Nat Neurosci. 2006. PMID: 16439983 Review.
-
The interaction between L1-type proteins and ankyrins--a master switch for L1-type CAM function.Cell Mol Biol Lett. 2009;14(1):57-69. doi: 10.2478/s11658-008-0035-4. Epub 2008 Oct 6. Cell Mol Biol Lett. 2009. PMID: 18839070 Free PMC article. Review.
Cited by
-
Ankyrins: Roles in synaptic biology and pathology.Mol Cell Neurosci. 2018 Sep;91:131-139. doi: 10.1016/j.mcn.2018.04.010. Epub 2018 May 3. Mol Cell Neurosci. 2018. PMID: 29730177 Free PMC article. Review.
-
Adolescent female C57BL/6 mice with vulnerability to activity-based anorexia exhibit weak inhibitory input onto hippocampal CA1 pyramidal cells.Neuroscience. 2013 Jun 25;241:250-67. doi: 10.1016/j.neuroscience.2013.03.020. Epub 2013 Mar 21. Neuroscience. 2013. PMID: 23523748 Free PMC article.
-
Intercellular protein-protein interactions at synapses.Protein Cell. 2014 Jun;5(6):420-44. doi: 10.1007/s13238-014-0054-z. Epub 2014 Apr 23. Protein Cell. 2014. PMID: 24756565 Free PMC article. Review.
-
Immunoglobulin-Like Receptors and Their Impact on Wiring of Brain Synapses.Annu Rev Genet. 2018 Nov 23;52:567-590. doi: 10.1146/annurev-genet-120417-031513. Epub 2018 Sep 13. Annu Rev Genet. 2018. PMID: 30212237 Free PMC article. Review.
-
Transsynaptic modality codes in the brain: possible involvement of synchronized spike timing, microRNAs, exosomes and epigenetic processes.Front Integr Neurosci. 2013 Jan 4;6:126. doi: 10.3389/fnint.2012.00126. eCollection 2012. Front Integr Neurosci. 2013. PMID: 23316146 Free PMC article.
References
-
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. - PubMed
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. - PubMed
-
- Bennett V, Chen L. Ankyrins and cellular targeting of diverse membrane proteins to physiological sites. Curr Opin Cell Biol. 2001;13:61–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

