Mixed GABA-glycine synapses delineate a specific topography in the nucleus tractus solitarii of adult rat
- PMID: 20156844
- PMCID: PMC2852998
- DOI: 10.1113/jphysiol.2009.184838
Mixed GABA-glycine synapses delineate a specific topography in the nucleus tractus solitarii of adult rat
Abstract
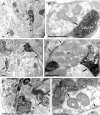
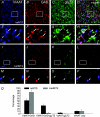
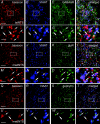
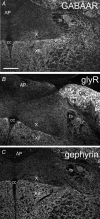
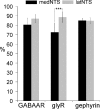
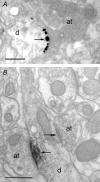
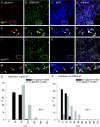
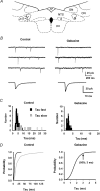
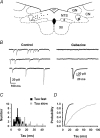
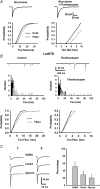
Using combined morphological and electrophysiological approaches, we have determined the composition of inhibitory synapses of the nucleus tractus solitarii (NTS), a brainstem structure that is a gateway for many visceral sensory afferent fibres. Immunohistochemical experiments demonstrate that, in adult rat, GABA axon terminals are present throughout the NTS while mixed GABA-glycine axon terminals are strictly located to the lateral part of the NTS within subnuclei surrounding the tractus solitarius. Purely glycine axon terminals are rare in the lateral part of the NTS and hardly detected in its medial part. Electrophysiological experiments confirm the predominance of GABA inhibition throughout the NTS and demonstrate the existence of a dual inhibition involving the co-release of GABA and glycine restricted to the lateral part of NTS. Since GABA(A) and glycine receptors are co-expressed postsynaptically in virtually all the inhibitory axon terminals throughout the NTS, it suggests that the inhibition phenotype relies on the characteristics of the axon terminals. Our results also demonstrate that glycine is mostly associated with GABA within axon terminals and raise the possibility of a dynamic regulation of GABA/glycine release at the presynaptic level. Our data provide new information for understanding the mechanisms involved in the processing of visceral information by the central nervous system in adult animals.
Figures

References
-
- Andresen MC, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius: common denominators. Chem Senses. 1996;21:387–395. - PubMed
-
- Bailey TW, Appleyard SM, Jin Y-H, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

