Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma
- PMID: 20156973
- PMCID: PMC2839147
- DOI: 10.1084/jem.20091921
Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma
Abstract
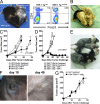
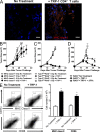
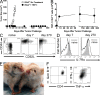
In vitro differentiated CD8(+) T cells have been the primary focus of immunotherapy of cancer with little focus on CD4(+) T cells. Immunotherapy involving in vitro differentiated T cells given after lymphodepleting regimens significantly augments antitumor immunity in animals and human patients with cancer. However, the mechanisms by which lymphopenia augments adoptive cell therapy and the means of properly differentiating T cells in vitro are still emerging. We demonstrate that naive tumor/self-specific CD4(+) T cells naturally differentiated into T helper type 1 cytotoxic T cells in vivo and caused the regression of established tumors and depigmentation in lymphopenic hosts. Therapy was independent of vaccination, exogenous cytokine support, CD8(+), B, natural killer (NK), and NKT cells. Proper activation of CD4(+) T cells in vivo was important for tumor clearance, as naive tumor-specific CD4(+) T cells could not completely treat tumor in lymphopenic common gamma chain (gamma(c))-deficient hosts. gamma(c) signaling in the tumor-bearing host was important for survival and proper differentiation of adoptively transferred tumor-specific CD4(+) T cells. Thus, these data provide a platform for designing immunotherapies that incorporate tumor/self-reactive CD4(+) T cells.
Figures

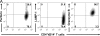

References
-
- Antony P.A., Piccirillo C.A., Akpinarli A., Finkelstein S.E., Speiss P.J., Surman D.R., Palmer D.C., Chan C.C., Klebanoff C.A., Overwijk W.W., et al. 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174:2591–2601 - PMC - PubMed
-
- Behrens G.M., Li M., Davey G.M., Allison J., Flavell R.A., Carbone F.R., Heath W.R. 2004b. Helper requirements for generation of effector CTL to islet beta cell antigens. J. Immunol. 172:5420–5426 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

