A structural determinant that renders G alpha(i) sensitive to activation by GIV/girdin is required to promote cell migration
- PMID: 20157114
- PMCID: PMC2857069
- DOI: 10.1074/jbc.M109.045161
A structural determinant that renders G alpha(i) sensitive to activation by GIV/girdin is required to promote cell migration
Abstract
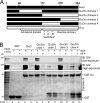
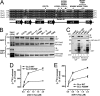
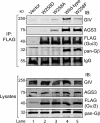
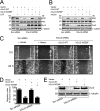
Although several non-receptor activators of heterotrimeric G proteins have been identified, the structural features of G proteins that determine their interaction with such activators and the subsequent biological effects are poorly understood. Here we investigated the structural determinants in G alpha(i3) necessary for its regulation by GIV/girdin, a guanine-nucleotide exchange factor (GEF) that activates G alpha(i) subunits. Using G protein activity and in vitro pulldown assays we demonstrate that G alpha(i3) is a better substrate for GIV than the highly homologous G alpha(o). We identified Trp-258 in the G alpha(i) subunit as a novel structural determinant for GIV binding by comparing GIV binding to G alpha(i3)/G alpha(o) chimeras. Mutation of Trp-258 to the corresponding Phe in G alpha(o) decreased GIV binding in vitro and in cultured cells but did not perturb interaction with other G alpha-binding partners, i.e. G betagamma, AGS3 (a guanine nucleotide dissociation inhibitor), GAIP/RGS19 (a GTPase-activating protein), and LPAR1 (a G protein-coupled receptor). Activation of G alpha(i3) by GIV was also dramatically reduced when Trp-258 was replaced with Tyr, Leu, Ser, His, Asp, or Ala, highlighting that Trp is required for maximal activation. Moreover, when mutant G alpha(i3) W258F was expressed in HeLa cells they failed to undergo cell migration and to enhance Akt signaling after growth factor or G protein-coupled receptor stimulation. Thus activation of G alpha(i3) by GIV is essential for biological functions associated with G alpha(i3) activation. In conclusion, we have discovered a novel structural determinant on G alpha(i) that plays a key role in defining the selectivity and efficiency of the GEF activity of GIV on G alpha(i) and that represents an attractive target site for designing small molecules to disrupt the G alpha(i)-GIV interface for therapeutic purposes.
Figures






References
-
- De Vries L., Zheng B., Fischer T., Elenko E., Farquhar M. G. (2000) Annu. Rev. Pharmacol. Toxicol. 40, 235–271 - PubMed
-
- Gilman A. G. (1987) Annu. Rev. Biochem. 56, 615–649 - PubMed
-
- Ross E. M., Wilkie T. M. (2000) Annu. Rev. Biochem. 69, 795–827 - PubMed
-
- Oldham W. M., Hamm H. E. (2008) Nat. Rev. Mol. Cell Biol. 9, 60–71 - PubMed
-
- Overington J. P., Al-Lazikani B., Hopkins A. L. (2006) Nat. Rev. Drug Discov. 5, 993–996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

