Cystatin C protects neuronal cells from amyloid-beta-induced toxicity
- PMID: 20157244
- PMCID: PMC2889175
- DOI: 10.3233/JAD-2010-1291
Cystatin C protects neuronal cells from amyloid-beta-induced toxicity
Abstract
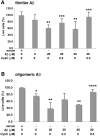
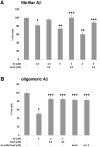
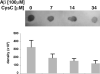
Multiple studies suggest that cystatin C (CysC) has a role in Alzheimer's disease (AD) and a decrease in CysC secretion is linked to the disease in patients with a polymorphism in the CysC gene. CysC binds amyloid-beta (Abeta) and inhibits formation of Abeta fibrils and oligomers both in vitro and in mouse models of amyloid deposition. Here we studied the effect of CysC on cultured primary hippocampal neurons and a neuronal cell line exposed to either oligomeric or fibrillar cytotoxic forms of Abeta. The extracellular addition of the secreted human CysC together with preformed either oligomeric or fibrillar Abeta increased cell survival. While CysC inhibits Abeta aggregation, it does not dissolve preformed Abeta fibrils or oligomers. Thus, CysC has multiple protective effects in AD, by preventing the formation of the toxic forms of Abeta and by direct protection of neuronal cells from Abeta toxicity. Therapeutic manipulation of CysC levels, resulting in slightly higher concentrations than physiological could protect neuronal cells from cell death in AD.
Figures

Similar articles
-
Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer's disease.Neuron. 2008 Oct 23;60(2):247-57. doi: 10.1016/j.neuron.2008.10.001. Neuron. 2008. PMID: 18957217 Free PMC article.
-
Cystatin C in Alzheimer's disease.Front Mol Neurosci. 2012 Jul 6;5:79. doi: 10.3389/fnmol.2012.00079. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22783166 Free PMC article.
-
Complexes of amyloid-beta and cystatin C in the human central nervous system.J Alzheimers Dis. 2009;18(2):273-80. doi: 10.3233/JAD-2009-1147. J Alzheimers Dis. 2009. PMID: 19584436 Free PMC article.
-
Cystatin C in aging and in Alzheimer's disease.Ageing Res Rev. 2016 Dec;32:38-50. doi: 10.1016/j.arr.2016.06.003. Epub 2016 Jun 19. Ageing Res Rev. 2016. PMID: 27333827 Free PMC article. Review.
-
The Positive Side of the Alzheimer's Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1.Molecules. 2020 May 23;25(10):2439. doi: 10.3390/molecules25102439. Molecules. 2020. PMID: 32456156 Free PMC article. Review.
Cited by
-
The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space.J Biol Chem. 2012 Dec 14;287(51):43108-15. doi: 10.1074/jbc.M112.404467. Epub 2012 Nov 5. J Biol Chem. 2012. PMID: 23129776 Free PMC article.
-
Protocol for High-Throughput Screening of Neural Cell or Brain Tissue Protein Using a Dot-Blot Technique with Near-Infrared Imaging.STAR Protoc. 2020 Sep 18;1(2):100054. doi: 10.1016/j.xpro.2020.100054. Epub 2020 Jun 13. STAR Protoc. 2020. PMID: 33043308 Free PMC article.
-
Induction of autophagy by cystatin C: a potential mechanism for prevention of cerebral vasospasm after experimental subarachnoid hemorrhage.Eur J Med Res. 2013 Jul 1;18(1):21. doi: 10.1186/2047-783X-18-21. Eur J Med Res. 2013. PMID: 23816364 Free PMC article.
-
Extracellular protein components of amyloid plaques and their roles in Alzheimer's disease pathology.Mol Neurodegener. 2021 Aug 28;16(1):59. doi: 10.1186/s13024-021-00465-0. Mol Neurodegener. 2021. PMID: 34454574 Free PMC article. Review.
-
Possible Mechanisms by which Stefin B could Regulate Proteostasis and Oxidative Stress.Cells. 2019 Jan 18;8(1):70. doi: 10.3390/cells8010070. Cells. 2019. PMID: 30669344 Free PMC article. Review.
References
-
- Maruyama K, Ikeda S, Ishihara T, Allsop D, Yanagisawa N. Immunohistochemical characterization of cerebrovascular amyloid in 46 autopsied cases using antibodies to β protein and cystatin C. Stroke. 1990;21:397–403. - PubMed
-
- Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J Neurol Sci. 1993;116:135–141. - PubMed
-
- Levy E, Sastre M, Kumar A, Gallo G, Piccardo P, Ghetti B, Tagliavini F. Codeposition of cystatin C with amyloid-ß protein in the brain of Alzheimer's disease patients. J Neuropathol Exp Neurol. 2001;60:94–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

