Leptin reduces the accumulation of Abeta and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices
- PMID: 20157255
- PMCID: PMC2924284
- DOI: 10.3233/JAD-2010-1298
Leptin reduces the accumulation of Abeta and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices
Abstract
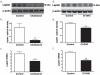
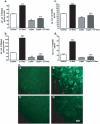
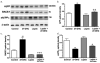
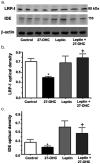
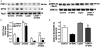
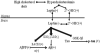
Accumulation of amyloid-beta (Abeta) peptide and deposition of hyperphosphorylated tau protein are two major pathological hallmarks of Alzheimer's disease (AD). We have shown that cholesterol-enriched diets and its metabolite 27-hydroxycholesterol (27-OHC) increase Abeta and phosphorylated tau levels. However, the mechanisms by which cholesterol and 27-OHC regulate Abeta production and tau phosphorylation remain unclear. Leptin, an adipocytokine involved in cell survival and in learning, has been demonstrated to regulate Abeta production and tau hyperphosphorylation in transgenic mice for AD. However, the involvement of leptin signaling in cholesterol and cholesterol metabolites-induced Abeta accumulation and tau hyperphosphorylation are yet to be examined. In this study, we determined the effect of high cholesterol diet and 27-OHC on leptin expression levels and the extent to which leptin treatment affects 27-OHC-induced AD-like pathology. Our results show that feeding rabbits a 2% cholesterol-enriched diet for 12 weeks reduces the levels of leptin by approximately 80% and incubating organotypic slices from adult rabbit hippocampus with 27-OHC reduced leptin levels by approximately 30%. 27-OHC induces a 1.5-fold increase in Abeta (40) and a 3-fold increase in Abeta (42) and in phosphorylated tau. Treatment with leptin reversed the 27-OHC-induced increase in Abeta and phosphorylated tau by decreasing the levels of BACE-1 and GSK-3beta respectively. Our results suggest that cholesterol-enriched diets and cholesterol metabolites induce AD-like pathology by altering leptin signaling. We propose that leptin administration may prevent the progression of sporadic forms of AD that are related to increased cholesterol and oxidized cholesterol metabolite levels.
Figures
References
-
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. - PubMed
-
- Wolozin B. Cholesterol and the biology of Alzheimer’s disease. Neuron. 2004;41:7–10. - PubMed
-
- Refolo LM, Pappolla MA, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K. Hypercholesterolemia upon differentiation associated increases in tau and cyclin-dependent kinase accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. - PubMed
-
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer’s disease beta-amyloid-peptides Aβ42 and Aβ40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

