Lysophosphatidylcholine Increases Ca Current via Activation of Protein Kinase C in Rabbit Portal Vein Smooth Muscle Cells
- PMID: 20157391
- PMCID: PMC2817530
- DOI: 10.4196/kjpp.2008.12.1.31
Lysophosphatidylcholine Increases Ca Current via Activation of Protein Kinase C in Rabbit Portal Vein Smooth Muscle Cells
Abstract
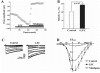
Lysophosphatidylcholine (LPC), a metabolite of membrane phospholipids by phospholipase A(2), has been considered responsible for the development of abnormal vascular reactivity during atherosclerosis. Ca(2+) influx was shown to be augmented in atherosclerotic artery which might be responsible for abnormal vascular reactivity. However, the mechanism underlying Ca(2+) influx change in atherosclerotic artery remains undetermined. The purpose of the present study was to examine the effects of LPC on L-type Ca(2+) current (I(Ca(L))) activity and to elucidate the mechanism of LPC-induced change of I(Ca(L)) in rabbit portal vein smooth muscle cells using whole cell patch clamp. Extracellular application of LPC increased I(Ca(L)) through whole test potentials, and this effect was readily reversed by washout. Steady state voltage dependency of activation or inactivation properties of I(Ca(L)) was not significantly changed by LPC. Staurosporine (100 nM) or chelerythrine (3 microM), which is a potent inhibitor of PKC, significantly decreased basal I(Ca(L)), and LPC-induced increase of I(Ca(L)) was significantly suppressed in the presence of PKC inhibitors. On the other hand, application of PMA, an activator of PKC, increased basal I(Ca(L)) significantly, and LPC-induced enhancement of I(Ca(L)) was abolished by pretreatment of the cells with PMA. These findings suggest that LPC increased I(Ca(L)) in vascular smooth muscle cells by a pathway that involves PKC, and that LPC-induced increase of I(Ca(L)) might be, at least in part, responsible for increased Ca(2+) influx in atherosclerotic artery.
Keywords: Ca2+ current; Lysophosphatidylcholine; Protein kinase C; Vascular smooth muscle.
Figures
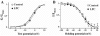

References
-
- Auge N, Fitoussi G, Bascand JL, Pieraggi M, Junquero D, Valet P. Mildly oxidized LDL evokes a sustained Ca2+-dependentretraction of vascular smooth muscle cells. Circ Res. 1996;79:871–880. - PubMed
-
- Chen Y, Morimoto S, Kitano S, Koh E, Fukuo K, Jiang B, Chen S, Yasuda O, Hirotani A, Ogihara T. Lysophosphatidylcholine causes Ca2+ influx, enhanced DNA synthesis and cytotoxicity incultured vascular smooth muscle cells. Atherosclerosis. 1995;112:69–76. - PubMed
-
- Cole WC, Clement-Chomienne O, Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem Cell Biol. 1996;74:439–447. - PubMed
-
- Cox DA, Cohen ML. Effects of oxidized low-density lipoprotein on vascular contraction and relaxation: clinical and pharmacological implications in atherosclerosis. Pharmacol Rev. 1996a;48:3–19. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

