Telomere length regulates ISG15 expression in human cells
- PMID: 20157543
- PMCID: PMC2806043
- DOI: 10.18632/aging.100066
Telomere length regulates ISG15 expression in human cells
Abstract
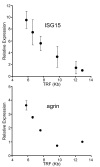
Endogenous genes regulated by telomere length have not previously been identified in human cells. Here we show that telomere length regulates the expression of interferon stimulated gene 15 (ISG15, 1p36.33). ISG15 expression (RNA and protein) increases in human cells with short telomeres, and decreases following the elongation of telomeres by human telomerase reverse transcriptase (hTERT). The short-telomere-dependent up-regulation of ISG15 is not mediated by replicative senescence/DNA damage signaling or type I interferons. In human skin specimens obtained from various aged individuals, ISG15 is up-regulated in a subset of cells in older individuals. Our results demonstrate that endogenous human genes can be regulated by the length of telomeres prior to the onset of DNA damage signals, and suggest the possibility that cell turnover/telomere shortening may provide a mechanism for adjusting cellular physiology. The upregulation of ISG15 with telomere shortening may contribute to chronic inflammatory states associated with human aging.
Keywords: ISG15; aging; cancer; cell turnover; inflammation; telomere position effect.
Conflict of interest statement
The authors declare they have no financial conflicts of interest.
Figures




References
-
- Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997;88:657–666. - PubMed
-
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
