Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment
- PMID: 20157580
- PMCID: PMC2815754
- DOI: 10.18632/aging.100105
Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment
Abstract
Of all the major organ systems in the body, the ovaries of females are the first to exhibit impaired function with advancing age. Until recently, traditional thinking was that female mammals are provided with a non-renewable pool of oocyte-containing follicles at birth that are depleted during postnatal life to exhaustion, driving ovarian failure. However, a growing body of evidence, including the isolation of germline stem cells (GSC) from adult mouse ovaries that produce developmentally-competent oocytes, has challenged this belief. In addition, rare germline stem-like cells capable of generating oocytes in vitro that undergo parthenogenesis to form blastocyst-like structures have recently been identified in postmenopausal human ovaries. Here we show that the germline-specific meiosis-commitment genes,Stimulated by retinoic acid gene 8 (Stra8) and Deleted in azoospermia-like (Dazl), are highly expressed in aged mouse ovaries. However, histological and marker analyses fail to demonstrate the presence of oocytes, supporting that Stra8 and Dazl are expressed in premeiotic germ cells that do not undergo further differentiation. Through the use of aged germline-specific GFP-expressing transgenic mice, we further show that these germ cells can generate GFP-positive oocytes that co-express the primordial oocyte marker NOBOX and form follicles when grafted into young adult wild-type female hosts. Thus, aged mouse ovaries possess a rare population of premeiotic germ cells that retain the capacity to form oocytes if exposed to a young host environment.
Keywords: Stra8; aging; germ cell; meiosis; oocyte; oogenesis; ovary; stem cell.
Conflict of interest statement
The authors of this manuscript have no conflict of interests to declare.
Figures


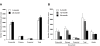

Comment in
-
Failure of the stem cell niche rather than loss of oocyte stem cells in the aging ovary.Aging (Albany NY). 2010 Jan 26;2(1):1-2. doi: 10.18632/aging.100119. Aging (Albany NY). 2010. PMID: 20228937 Free PMC article. Review. No abstract available.
References
-
- Buckler H. The menopause transition: endocrine changes and clinical symptoms. J Br Menopause Soc. 2005;11:61–65. - PubMed
-
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. - PubMed
-
- Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–109.
-
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, Wu J. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
