Dual regulation of TERT activity through transcription and splicing by DeltaNP63alpha
- PMID: 20157588
- PMCID: PMC2815765
- DOI: 10.18632/aging.100003
Dual regulation of TERT activity through transcription and splicing by DeltaNP63alpha
Abstract
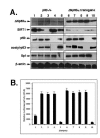
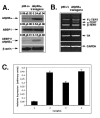
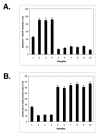
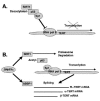
P53 homolog p63 was shown to play a role in premature ageing phenotype found in mouse models through regulation of the replicative senescence. We previously showed that the forced DeltaNp63alpha expression decreased the SIRT1 protein levels, and induced the replicative senescence of human keratinocytes, while the ectopic SIRT1 expression decreased the senescence. Using the DeltaNp63alpha overexpressing and p63-/+ heterozygous mice, we found that DeltaNp63alpha induced the mTERT promoter activation through the down regulation of the SIRT1 protein levels, inactivation of p53 deacetylation, decrease of the p53/Sp1 protein-protein interaction, and the overall induction of mTERT transcription regulation. In the same time, by a forming of protein-protein complexes with the ABBP1, DeltaNp63alpha induced the mTERT RNA splicing leading to an increasing expression of spliced mTERT isoforms playing a role of dominant-negative inhibitors of mTERT activity and therefore decreasing the levels of TERT activity in mouse epidermal keratinocytes. The overall effect of the DeltaNp63alpha overexpression resulted in decrease in telomerase activity and increase in replicative senescence observed in mouse keratinocytes. This dual molecular mechanism of telomerase regulation might underline the previously shown effect of DeltaNp63alpha on premature ageing phenotype.
Keywords: P63; SIRT1; Sp1; TERT; ageing; mouse; p53; senescence; splicing; transcription.
Conflict of interest statement
The authors of this manuscript have no conflict of interests to declare.
Figures


Similar articles
-
Dysregulated ΔNp63α negatively regulates the maspin promoter in keratinocytes via blocking endogenous p73 binding.Mol Carcinog. 2014 Sep;53(9):698-710. doi: 10.1002/mc.22022. Epub 2013 Mar 8. Mol Carcinog. 2014. PMID: 23475637
-
p63 directly induces expression of Alox12, a regulator of epidermal barrier formation.Exp Dermatol. 2009 Dec;18(12):1016-21. doi: 10.1111/j.1600-0625.2009.00894.x. Exp Dermatol. 2009. PMID: 19555433 Free PMC article.
-
DeltaNp63alpha repression of the Notch1 gene supports the proliferative capacity of normal human keratinocytes and cervical cancer cells.Cancer Res. 2010 May 15;70(10):4034-44. doi: 10.1158/0008-5472.CAN-09-4063. Epub 2010 May 4. Cancer Res. 2010. PMID: 20442293
-
Molecular regulation of telomerase activity in aging.Protein Cell. 2011 Sep;2(9):726-38. doi: 10.1007/s13238-011-1093-3. Epub 2011 Oct 6. Protein Cell. 2011. PMID: 21976062 Free PMC article. Review.
-
P63 in health and cancer.Int J Dev Biol. 2015;59(1-3):87-93. doi: 10.1387/ijdb.150045sg. Int J Dev Biol. 2015. PMID: 26374530 Review.
Cited by
-
Medical genetics and epigenetics of telomerase.J Cell Mol Med. 2011 Mar;15(3):457-67. doi: 10.1111/j.1582-4934.2011.01276.x. J Cell Mol Med. 2011. PMID: 21323862 Free PMC article. Review.
-
Sirt1: def-eating senescence?Cell Cycle. 2012 Nov 15;11(22):4135-46. doi: 10.4161/cc.22074. Epub 2012 Sep 14. Cell Cycle. 2012. PMID: 22983125 Free PMC article. Review.
-
Distinct p63 and p73 Protein Interactions Predict Specific Functions in mRNA Splicing and Polyploidy Control in Epithelia.Cells. 2020 Dec 25;10(1):25. doi: 10.3390/cells10010025. Cells. 2020. PMID: 33375680 Free PMC article. Review.
-
Research advances and treatment perspectives of pancreatic adenosquamous carcinoma.Cell Oncol (Dordr). 2023 Feb;46(1):1-15. doi: 10.1007/s13402-022-00732-2. Epub 2022 Nov 1. Cell Oncol (Dordr). 2023. PMID: 36316580
-
Telomere shortening induces aging-associated phenotypes in hiPSC-derived neurons and astrocytes.Biogerontology. 2024 Apr;25(2):341-360. doi: 10.1007/s10522-023-10076-5. Epub 2023 Nov 21. Biogerontology. 2024. PMID: 37987889 Free PMC article.
References
-
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. - PubMed
-
- Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–3361. - PubMed
-
- Greer EL, Brunet A. Signaling networks in ageing. J Cell Sci. 2008;121:407–412. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
