An evaluation of the factors that affect the health-related quality of life of children following myelosuppressive chemotherapy
- PMID: 20157746
- PMCID: PMC2909452
- DOI: 10.1007/s00520-010-0824-y
An evaluation of the factors that affect the health-related quality of life of children following myelosuppressive chemotherapy
Abstract
Purpose: The purposes of this study, in children who were assessed 1 week after the administration of myelosuppressive chemotherapy were: to compare the total and subscale scores on a generic measure of health-related quality of life (HRQOL) to normative data from healthy children and describe the relationships between demographic, clinical, and symptom characteristics of children with cancer and generic and disease-specific dimensions of HRQOL.
Methods: Patients (n = 61) were predominantly male (52.5%), minority (63.9%), and 14.7 years of age. Children completed the Memorial Symptom Assessment Scale for 10- to 18-year olds, the PedsQL™ Generic and Cancer Modules, and the Karnofsky Performance Status (KPS) scale 1 week after the start of a chemotherapy cycle.
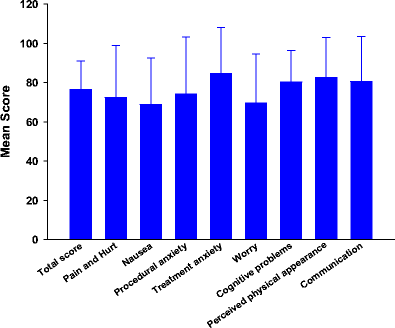
Results: The mean number of symptoms per patient was 10.6. Compared with the normative sample, children with cancer reported significantly lower scores for the total scale and all of the subscales except emotional and social functioning. No significant differences were found between any demographic characteristics and total or subscale scores on the generic or disease-specific measures of HRQOL. Lower KPS scores were associated with poorer generic and disease-specific HRQOL scores. In addition, a higher number of symptoms was associated with poorer generic and disease-specific HRQOL scores. Finally, higher symptom distress scores were associated with poorer generic and disease-specific HRQOL scores.
Conclusion: Among the demographic, clinical, and symptom characteristics studied, poorer functional status and higher symptom burden were associated with significant decreases in HRQOL in children who received myelosuppressive chemotherapy.
Figures
Similar articles
-
Changes in children's reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy.J Pediatr Oncol Nurs. 2010 Nov-Dec;27(6):307-15. doi: 10.1177/1043454210377619. Epub 2010 Aug 25. J Pediatr Oncol Nurs. 2010. PMID: 20739586
-
Epilepsy in patients with primary brain tumors: The impact on mood, cognition, and HRQOL.Epilepsy Behav. 2015 Jul;48:88-95. doi: 10.1016/j.yebeh.2015.03.016. Epub 2015 Jun 29. Epilepsy Behav. 2015. PMID: 26136184
-
Use of the Pediatric Quality of Life Inventory to assess the health-related quality of life in children with recurrent respiratory papillomatosis.Ann Otol Rhinol Laryngol. 2005 Jul;114(7):499-503. doi: 10.1177/000348940511400701. Ann Otol Rhinol Laryngol. 2005. PMID: 16134343
-
Explaining parent-child (dis)agreement in generic and short stature-specific health-related quality of life reports: do family and social relationships matter?Health Qual Life Outcomes. 2016 Oct 21;14(1):150. doi: 10.1186/s12955-016-0553-0. Health Qual Life Outcomes. 2016. PMID: 27769269 Free PMC article.
-
Health-related quality of life for pediatric patients with end-stage kidney disease: A systematic review and meta-analysis of the Pediatric Quality of Life Inventory (PedsQL).Hemodial Int. 2024 Apr;28(2):198-215. doi: 10.1111/hdi.13138. Epub 2024 Mar 11. Hemodial Int. 2024. PMID: 38468403
Cited by
-
Frequency, Severity, and Distress Associated With Physical and Psychosocial Symptoms at Home in Children and Adolescents With Cancer.J Pediatr Health Care. 2019 Jul-Aug;33(4):404-414. doi: 10.1016/j.pedhc.2018.11.007. Epub 2019 Mar 5. J Pediatr Health Care. 2019. PMID: 30846334 Free PMC article.
-
Symptoms in Children Receiving Treatment for Cancer-Part I: Fatigue, Sleep Disturbance, and Nausea/Vomiting.J Pediatr Oncol Nurs. 2019 Jul/Aug;36(4):244-261. doi: 10.1177/1043454219849576. J Pediatr Oncol Nurs. 2019. PMID: 31307321 Free PMC article. Review.
-
Fatigue and health related quality of life in children and adolescents with cancer.Eur J Oncol Nurs. 2017 Aug;29:39-46. doi: 10.1016/j.ejon.2017.05.001. Epub 2017 May 13. Eur J Oncol Nurs. 2017. PMID: 28720264 Free PMC article.
-
Exploring core symptoms and interrelationships among symptoms in children with acute leukemia during chemotherapy: A network analysis.Support Care Cancer. 2023 Sep 16;31(10):578. doi: 10.1007/s00520-023-08024-7. Support Care Cancer. 2023. PMID: 37715817
-
Perspectives of Childhood Cancer Symptom-Related Distress: Results of the State of the Science Survey.J Pediatr Oncol Nurs. 2019 Jul/Aug;36(4):287-293. doi: 10.1177/1043454219858608. J Pediatr Oncol Nurs. 2019. PMID: 31307322 Free PMC article.
References
-
- US Department of Health and Human Services FDA Center for Drug Evaluation and Research. US Department of Health and Human Services FDA Center for Biologics Evaluation and Research. US Department of Health and Human Services FDA Center for Devices and Radiological Health . Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. Silver Spring: Food and Drug Administration; 2006. - PubMed