Measuring the energetics of membrane protein dimerization in mammalian membranes
- PMID: 20158179
- PMCID: PMC3860826
- DOI: 10.1021/ja910692u
Measuring the energetics of membrane protein dimerization in mammalian membranes
Abstract
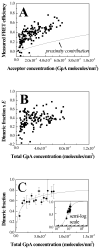
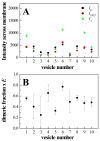
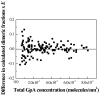
Thus far, quantitative studies of lateral protein interactions in membranes have been restricted peptides or simplified protein constructs in lipid vesicles or bacterial membranes. Here we show how free energies of membrane protein dimerization can be measured in mammalian plasma membrane-derived vesicles. The measurements, performed in single vesicles, utilize the quantitative imaging FRET (QI-FRET) method. The experiments are described in a step-by-step protocol. The protein characterized is the transmembrane domain of glycophorin A, the most extensively studied membrane protein, known to form homodimers in hydrophobic environments. The results suggest that molecular crowding in cellular membranes has a dramatic effect on the strength of membrane protein interactions.
Figures
Similar articles
-
Characterization of membrane protein interactions in plasma membrane derived vesicles with quantitative imaging Förster resonance energy transfer.Acc Chem Res. 2015 Aug 18;48(8):2262-9. doi: 10.1021/acs.accounts.5b00238. Epub 2015 Aug 5. Acc Chem Res. 2015. PMID: 26244699 Free PMC article.
-
Glycophorin A transmembrane domain dimerization in plasma membrane vesicles derived from CHO, HEK 293T, and A431 cells.Biochim Biophys Acta. 2013 Aug;1828(8):1829-33. doi: 10.1016/j.bbamem.2013.03.022. Epub 2013 Apr 2. Biochim Biophys Acta. 2013. PMID: 23562404 Free PMC article.
-
Changes in apparent free energy of helix-helix dimerization in a biological membrane due to point mutations.J Mol Biol. 2007 Aug 10;371(2):422-34. doi: 10.1016/j.jmb.2007.05.026. Epub 2007 May 18. J Mol Biol. 2007. PMID: 17570394 Free PMC article.
-
Not just an oil slick: how the energetics of protein-membrane interactions impacts the function and organization of transmembrane proteins.Biophys J. 2014 Jun 3;106(11):2305-16. doi: 10.1016/j.bpj.2014.04.032. Biophys J. 2014. PMID: 24896109 Free PMC article. Review.
-
Investigations of membrane protein interactions in cells using fluorescence microscopy.Curr Opin Struct Biol. 2024 Jun;86:102816. doi: 10.1016/j.sbi.2024.102816. Epub 2024 Apr 21. Curr Opin Struct Biol. 2024. PMID: 38648680 Free PMC article. Review.
Cited by
-
Characterization of membrane protein interactions in plasma membrane derived vesicles with quantitative imaging Förster resonance energy transfer.Acc Chem Res. 2015 Aug 18;48(8):2262-9. doi: 10.1021/acs.accounts.5b00238. Epub 2015 Aug 5. Acc Chem Res. 2015. PMID: 26244699 Free PMC article.
-
Probing macromolecular crowding at the lipid membrane interface with genetically-encoded sensors.Protein Sci. 2023 Nov;32(11):e4797. doi: 10.1002/pro.4797. Protein Sci. 2023. PMID: 37779215 Free PMC article.
-
Fully quantified spectral imaging reveals in vivo membrane protein interactions.Integr Biol (Camb). 2016 Feb;8(2):216-29. doi: 10.1039/c5ib00202h. Epub 2016 Jan 20. Integr Biol (Camb). 2016. PMID: 26787445 Free PMC article.
-
Martini 3: a general purpose force field for coarse-grained molecular dynamics.Nat Methods. 2021 Apr;18(4):382-388. doi: 10.1038/s41592-021-01098-3. Epub 2021 Mar 29. Nat Methods. 2021. PMID: 33782607
-
Neural network strategies for plasma membrane selection in fluorescence microscopy images.Biophys J. 2021 Jun 15;120(12):2374-2385. doi: 10.1016/j.bpj.2021.04.030. Epub 2021 May 4. Biophys J. 2021. PMID: 33961865 Free PMC article.
References
-
- Ellis RJ. Trends Biochem Sci. 2001;26:597–604. - PubMed
-
- Ellis RJ. Cur Opinion Struc Biol. 2001;11:114–19. - PubMed
-
- Minton AP. Cur Opinion Struc Biol. 2000;10:34–39. - PubMed
-
- MacKenzie KR. Chem Rev. 2006;106:1931–77. - PubMed
-
- Hong H, Joh NH, Bowie JU, Tamm LK. Methods in Enzymology: Biothermodynamics. 2009;455455(Part A):213–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

