Epoxyeicosatrienoic acid analogs and vascular function
- PMID: 20158473
- PMCID: PMC2855336
- DOI: 10.2174/092986710790827843
Epoxyeicosatrienoic acid analogs and vascular function
Abstract
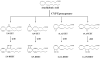
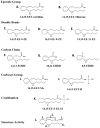
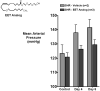
Arachidonic acid metabolites, eicosanoids, are key contributors to vascular function and improper eicosanoid regulation contributes to the progression of cardiovascular diseases. Epoxyeicosatrienoic acids (EETs) are synthesized from arachidonic acid by epoxygenase enzymes to four regioisomers, 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET. These EETs have interesting beneficial effects like vasodilation, anti-inflammation, and anti-platelet aggregation that could combat cardiovascular diseases. There is mounting evidence that each regioisomeric EET may have unique vascular effects and that the contribution of individual EETs to vascular function differs from organ to organ. Over the past decade EET analogs and antagonists have been synthesized to determine EET structure function relationships and define the contribution of each regioisomeric EET. A number of studies have demonstrated that EET analogs induce vasodilation, lower blood pressure and decrease inflammation. EET antagonists have also been used to demonstrate that endogenous EETs contribute importantly to cardiovascular function. This review will discuss EET synthesis, regulation and physiological roles in the cardiovascular system. Next we will focus on the development of EET analogs and what has been learned about their contribution to vascular function. Finally, the development of EET antagonists and how these have been utilized to determine the cardiovascular actions of endogenous epoxides will be discussed. Overall, this review will highlight the important knowledge garnered by the development of EET analogs and their possible value in the treatment of cardiovascular diseases.
Figures
Similar articles
-
Orally active epoxyeicosatrienoic acid analogs in hypertension and renal injury.Adv Pharmacol. 2022;94:27-55. doi: 10.1016/bs.apha.2022.02.004. Epub 2022 Mar 30. Adv Pharmacol. 2022. PMID: 35659375 Free PMC article.
-
Epoxides and soluble epoxide hydrolase in cardiovascular physiology.Physiol Rev. 2012 Jan;92(1):101-30. doi: 10.1152/physrev.00021.2011. Physiol Rev. 2012. PMID: 22298653 Free PMC article. Review.
-
Orally Active Epoxyeicosatrienoic Acid Analogs.J Cardiovasc Pharmacol. 2017 Oct;70(4):211-224. doi: 10.1097/FJC.0000000000000523. J Cardiovasc Pharmacol. 2017. PMID: 28937442 Free PMC article. Review.
-
The Role of Epoxyeicosatrienoic Acids in Cardiac Remodeling.Front Physiol. 2021 Feb 24;12:642470. doi: 10.3389/fphys.2021.642470. eCollection 2021. Front Physiol. 2021. PMID: 33716791 Free PMC article. Review.
-
Reno-protective mechanisms of epoxyeicosatrienoic acids in cardiovascular disease.Am J Physiol Regul Integr Comp Physiol. 2012 Feb 1;302(3):R321-30. doi: 10.1152/ajpregu.00606.2011. Epub 2011 Nov 23. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22116511 Review.
Cited by
-
Evaluation of the Therapeutic Potential of Sulfonyl Urea Derivatives as Soluble Epoxide Hydrolase (sEH) Inhibitors.Molecules. 2024 Jun 26;29(13):3036. doi: 10.3390/molecules29133036. Molecules. 2024. PMID: 38998987 Free PMC article.
-
Inhibiting an Epoxide Hydrolase Virulence Factor from Pseudomonas aeruginosa Protects CFTR.Angew Chem Int Ed Engl. 2015 Aug 17;54(34):9881-5. doi: 10.1002/anie.201503983. Epub 2015 Jul 1. Angew Chem Int Ed Engl. 2015. PMID: 26136396 Free PMC article.
-
Increased Soluble Epoxide Hydrolase Activity Positively Correlates with Mortality in Heart Failure Patients with Preserved Ejection Fraction: Evidence from Metabolomics.Phenomics. 2022 Oct 27;3(1):34-49. doi: 10.1007/s43657-022-00069-8. eCollection 2023 Feb. Phenomics. 2022. PMID: 36939801 Free PMC article.
-
Current Knowledge on the Role of Cardiolipin Remodeling in the Context of Lipid Oxidation and Barth Syndrome.Front Mol Biosci. 2022 May 27;9:915301. doi: 10.3389/fmolb.2022.915301. eCollection 2022. Front Mol Biosci. 2022. PMID: 35693555 Free PMC article.
-
Arachidonic acid metabolism in health and disease.MedComm (2020). 2023 Sep 20;4(5):e363. doi: 10.1002/mco2.363. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37746665 Free PMC article. Review.
References
-
- Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–9. - PMC - PubMed
-
- Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–62. - PubMed
-
- Daikh BE, Lasker JM, Raucy JL, Koop DR. Regio- and stereoselective epoxidation of arachidonic acid by human cytochromes P450 2C8 and 2C9. J Pharmacol Exp Ther. 1994;271:1427–33. - PubMed
-
- Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol. 1996;270:F822–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

