The role of caspase-2 in stress-induced apoptosis
- PMID: 20158568
- PMCID: PMC3828840
- DOI: 10.1111/j.1582-4934.2010.01037.x
The role of caspase-2 in stress-induced apoptosis
Abstract
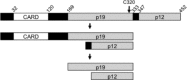
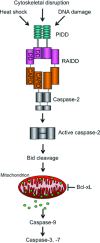
Caspase-2 is the most evolutionarily conserved of all the caspases, yet it has a poorly defined role in apoptotic pathways. This is mainly due to a dearth of techniques to determine the activation status of caspase-2 and the lack of an abnormal phenotype in caspase-2 deficient mice. Nevertheless, emerging evidence suggests that caspase-2 may have important functions in a number of stress-induced cell death pathways, in cell cycle maintenance and regulation of tumour progression. This review discusses recent advances that have been made to help elucidate the true role of this elusive caspase and the potential contribution of caspase-2 to the pathology of human diseases including cancer.
Figures
Similar articles
-
Caspase-2: controversial killer or checkpoint controller?Apoptosis. 2009 Jul;14(7):829-48. doi: 10.1007/s10495-009-0365-3. Apoptosis. 2009. PMID: 19479377 Review.
-
Caspase 2 in apoptosis, the DNA damage response and tumour suppression: enigma no more?Nat Rev Cancer. 2009 Dec;9(12):897-903. doi: 10.1038/nrc2745. Epub 2009 Nov 5. Nat Rev Cancer. 2009. PMID: 19890334 Review.
-
Caspase-2 as a tumour suppressor.Cell Death Differ. 2013 Sep;20(9):1133-9. doi: 10.1038/cdd.2013.87. Epub 2013 Jun 28. Cell Death Differ. 2013. PMID: 23811850 Free PMC article. Review.
-
The p53-caspase-2 axis in the cell cycle and DNA damage response.Exp Mol Med. 2021 Apr;53(4):517-527. doi: 10.1038/s12276-021-00590-2. Epub 2021 Apr 14. Exp Mol Med. 2021. PMID: 33854186 Free PMC article. Review.
-
Requirement of Apaf-1 for mitochondrial events and the cleavage or activation of all procaspases during genotoxic stress-induced apoptosis.Biochem J. 2007 Jul 1;405(1):115-22. doi: 10.1042/BJ20061576. Biochem J. 2007. PMID: 17348858 Free PMC article.
Cited by
-
Deep profiling of protease substrate specificity enabled by dual random and scanned human proteome substrate phage libraries.Proc Natl Acad Sci U S A. 2020 Oct 13;117(41):25464-25475. doi: 10.1073/pnas.2009279117. Epub 2020 Sep 24. Proc Natl Acad Sci U S A. 2020. PMID: 32973096 Free PMC article.
-
Inhibition of Caspase-2 Translation by the mRNA Binding Protein HuR: A Novel Path of Therapy Resistance in Colon Carcinoma Cells?Cells. 2019 Jul 30;8(8):797. doi: 10.3390/cells8080797. Cells. 2019. PMID: 31366165 Free PMC article. Review.
-
Mechanistic Interplay Between Autophagy and Apoptotic Signaling in Endosulfan-Induced Dopaminergic Neurotoxicity: Relevance to the Adverse Outcome Pathway in Pesticide Neurotoxicity.Toxicol Sci. 2019 Jun 1;169(2):333-352. doi: 10.1093/toxsci/kfz049. Toxicol Sci. 2019. PMID: 30796443 Free PMC article.
-
The IRE1α Stress Signaling Axis Is a Key Regulator of Neutrophil Antimicrobial Effector Function.J Immunol. 2021 Jul 1;207(1):210-220. doi: 10.4049/jimmunol.2001321. Epub 2021 Jun 18. J Immunol. 2021. PMID: 34145058 Free PMC article.
-
S6K1 is a Targetable Vulnerability in Tumors Exhibiting Plasticity and Therapy Resistance.Int J Biol Sci. 2025 Jan 1;21(2):454-472. doi: 10.7150/ijbs.96672. eCollection 2025. Int J Biol Sci. 2025. PMID: 39781466 Free PMC article.
References
-
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–41. - PubMed
-
- Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003;193:10–21. - PubMed
-
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

