MafA promotes the reprogramming of placenta-derived multipotent stem cells into pancreatic islets-like and insulin+ cells
- PMID: 20158571
- PMCID: PMC3922383
- DOI: 10.1111/j.1582-4934.2010.01034.x
MafA promotes the reprogramming of placenta-derived multipotent stem cells into pancreatic islets-like and insulin+ cells
Abstract
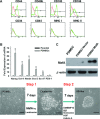
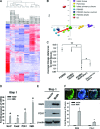
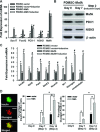
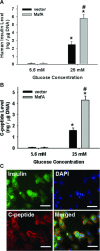
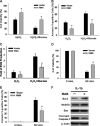
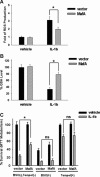
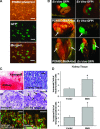
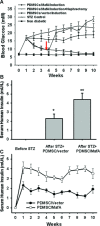
MafA is a pancreatic transcriptional factor that controls β-cell-specific transcription of the insulin gene. However, the role of MafA in the regulation of pancreatic transdifferentiation and reprogramming in human stem cells is still unclear. In this study, we investigate the role of MafA in placenta-derived multipotent stem cells (PDMSCs) that constitutively expressed Oct-4 and Nanog. PDMSCs were isolated and transfected with MafA using a lentivector. Our results showed that overexpression of MafA in PDMSCs significantly up-regulated the expression of pancreatic development-related genes (Sox17, Foxa2, Pdx1 and Ngn3). Microarray analysis suggested that the gene expression profile of MafA-overexpressing PDMSCs was similar to that of pancreas and islet tissues. MafA increased the expression levels of the mRNAs of NKx2.2, Glut2, insulin, glucagons and somatostatin, and further facilitated the differentiation of PDMSCs into insulin(+) cells. The glucose-stimulated responses to insulin and c-peptide production in MafA-overexpressing PDMSCs were significantly higher than in PDMSCs with vector control. Our results indicated that MafA-overexpressing PDMSCs were more resistant to oxidative damage and oxidative damage-induced apoptosis than PDMSCs carrying the vector control were. Importantly, the expression of MafA in PDMSCs xenotransplanted into immunocompromised mice improved the restoration of blood insulin levels to control values and greatly prolonged the survival of graft cells in immunocompromised mice with STZ-induced diabetes. In summary, these data suggest that MafA plays a novel role in the reprogramming of stem cells into pancreatic β-progenitors, promotes the islet-like characteristics of PDMSCs, as well as functionally enhances insulin production to restore the regulation of blood glucose levels in transplanted grafts.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
Similar articles
-
TAT-mediated transduction of MafA protein in utero results in enhanced pancreatic insulin expression and changes in islet morphology.PLoS One. 2011;6(8):e22364. doi: 10.1371/journal.pone.0022364. Epub 2011 Aug 4. PLoS One. 2011. PMID: 21857924 Free PMC article.
-
Placenta-derived multipotent stem cells induced to differentiate into insulin-positive cells.Biochem Biophys Res Commun. 2007 Jun 1;357(2):414-20. doi: 10.1016/j.bbrc.2007.03.157. Epub 2007 Apr 3. Biochem Biophys Res Commun. 2007. PMID: 17433254
-
Induction of insulin-producing cells derived from endometrial mesenchymal stem-like cells.J Pharmacol Exp Ther. 2010 Dec;335(3):817-29. doi: 10.1124/jpet.110.169284. Epub 2010 Sep 20. J Pharmacol Exp Ther. 2010. PMID: 20855446
-
PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
-
Roles and regulation of transcription factor MafA in islet beta-cells.Endocr J. 2007 Dec;54(5):659-66. doi: 10.1507/endocrj.kr-101. Epub 2007 Aug 30. Endocr J. 2007. PMID: 17785922 Review.
Cited by
-
Inhibition of phosphorylated STAT3 by cucurbitacin I enhances chemoradiosensitivity in medulloblastoma-derived cancer stem cells.Childs Nerv Syst. 2012 Mar;28(3):363-73. doi: 10.1007/s00381-011-1672-x. Epub 2012 Jan 17. Childs Nerv Syst. 2012. PMID: 22249380
-
TAT-mediated transduction of MafA protein in utero results in enhanced pancreatic insulin expression and changes in islet morphology.PLoS One. 2011;6(8):e22364. doi: 10.1371/journal.pone.0022364. Epub 2011 Aug 4. PLoS One. 2011. PMID: 21857924 Free PMC article.
-
MafA Overexpression: A New Efficient Protocol for In Vitro Differentiation of Adipose-Derived Mesenchymal Stem Cells into Functional Insulin-Producing Cells.Cell J. 2019 Jul;21(2):169-178. doi: 10.22074/cellj.2019.5669. Epub 2019 Feb 20. Cell J. 2019. PMID: 30825290 Free PMC article.
-
Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus.Oxid Med Cell Longev. 2020 Mar 9;2020:8609213. doi: 10.1155/2020/8609213. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32215179 Free PMC article. Review.
-
Stem Cells Reprogramming in Diabetes Mellitus and Diabetic Complications: Recent Advances.Curr Diabetes Rev. 2025;21(4):21-37. doi: 10.2174/0115733998275428231210055650. Curr Diabetes Rev. 2025. PMID: 38173073 Review.
References
-
- Ryan EA, Lakey JR, Paty BW, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–57. - PubMed
-
- Robertson RP. Islet transplantation as a treatment for diabetes – a work in progress. N Engl J Med. 2004;350:694–705. - PubMed
-
- Kaneto H, Matsuoka TA, Kawashima S, et al. Role of MafA in pancreatic beta-cells. Adv Drug Deliv Rev. 2009;61:489–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

