Enhanced platelet adhesion induces angiogenesis in intestinal inflammation and inflammatory bowel disease microvasculature
- PMID: 20158572
- PMCID: PMC3922384
- DOI: 10.1111/j.1582-4934.2010.01033.x
Enhanced platelet adhesion induces angiogenesis in intestinal inflammation and inflammatory bowel disease microvasculature
Abstract
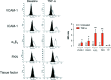
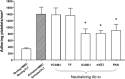
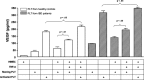
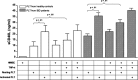
Although angiogenesis is viewed as a fundamental component of inflammatory bowel disease (IBD) pathogenesis, we presently lack a thorough knowledge of the cell type(s) involved in its induction and maintenance in the inflamed intestinal mucosa. This study aimed to determine whether platelet (PLT) adhesion to inflamed intestinal endothelial cells of human origin may favour angiogenesis. Unstimulated or thrombin-activated human PLT were overlaid on resting or tumour necrosis factor (TNF)-α-treated human intestinal microvascular endothelial cells (HIMEC), in the presence or absence of blocking antibodies to either vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1, integrin α(v)β(3) , tissue factor (TF) or fractalkine (FKN). PLT adhesion to HIMEC was evaluated by fluorescence microscopy, and release of angiogenic factors (VEGF and soluble CD40L) was measured by ELISA. A matrigel tubule formation assay was used to estimate PLT capacity to induce angiogenesis after co-culturing with HIMEC. TNF-α up-regulated ICAM-1, α(v)β(3) and FKN expression on HIMEC. When thrombin-activated PLT were co-cultured with unstimulated HIMEC, PLT adhesion increased significantly, and this response was further enhanced by HIMEC activation with TNF-α. PLT adhesion to HIMEC was VCAM-1 and TF independent but ICAM-1, FKN and integrin α(v)β(3) dependent. VEGF and sCD40L were undetectable in HIMEC cultures either before or after TNF-α stimulation. By contrast, VEGF and sCD40L release significantly increased when resting or activated PLT were co-cultured with TNF-α-pre-treated HIMEC. These effects were much more pronounced when PLT were derived from IBD patients. Importantly, thrombin-activated PLT promoted tubule formation in HIMEC, a functional estimate of their angiogenic potential. In conclusion, PLT adhesion to TNF-α-pre-treated HIMEC is mediated by ICAM-1, FKN and α(v)β(3) , and is associated with VEGF and sCD40L release. These findings suggest that inflamed HIMEC may recruit PLT which, upon release of pro-angiogenic factors, actively contribute to inflammation-induced angiogenesis.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

Similar articles
-
Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients.Gastroenterology. 2003 May;124(5):1249-64. doi: 10.1016/s0016-5085(03)00289-0. Gastroenterology. 2003. PMID: 12730866
-
Thalidomide inhibits inflammatory and angiogenic activation of human intestinal microvascular endothelial cells (HIMEC).Am J Physiol Gastrointest Liver Physiol. 2010 Feb;298(2):G167-76. doi: 10.1152/ajpgi.00385.2009. Epub 2009 Nov 19. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 19926820 Free PMC article.
-
TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn's disease.J Immunol. 2006 Feb 15;176(4):2617-24. doi: 10.4049/jimmunol.176.4.2617. J Immunol. 2006. PMID: 16456024 Clinical Trial.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Role of the endothelium in inflammatory bowel diseases.World J Gastroenterol. 2011 Feb 7;17(5):578-93. doi: 10.3748/wjg.v17.i5.578. World J Gastroenterol. 2011. PMID: 21350707 Free PMC article. Review.
Cited by
-
Biomarkers in inflammatory bowel disease: current practices and recent advances.Transl Res. 2012 Apr;159(4):313-25. doi: 10.1016/j.trsl.2012.01.001. Epub 2012 Feb 1. Transl Res. 2012. PMID: 22424434 Free PMC article. Review.
-
The Inflammatory Role of Platelets via Their TLRs and Siglec Receptors.Front Immunol. 2015 Mar 2;6:83. doi: 10.3389/fimmu.2015.00083. eCollection 2015. Front Immunol. 2015. PMID: 25784910 Free PMC article. Review.
-
Stem Cell-Based Therapies for Inflammatory Bowel Disease.Int J Mol Sci. 2022 Jul 31;23(15):8494. doi: 10.3390/ijms23158494. Int J Mol Sci. 2022. PMID: 35955628 Free PMC article. Review.
-
Analysis of the interferon-γ-induced secretome of intestinal endothelial cells: putative impact on epithelial barrier dysfunction in IBD.Front Cell Dev Biol. 2023 Aug 14;11:1213383. doi: 10.3389/fcell.2023.1213383. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37645250 Free PMC article.
-
Inhibition of Angiogenesis and Effect on Inflammatory Bowel Disease of Ginsenoside Rg3-Loaded Thermosensitive Hydrogel.Pharmaceutics. 2024 Sep 25;16(10):1243. doi: 10.3390/pharmaceutics16101243. Pharmaceutics. 2024. PMID: 39458575 Free PMC article.
References
-
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–63. - PubMed
-
- Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

