Chemokine-cleaving Streptococcus pyogenes protease SpyCEP is necessary and sufficient for bacterial dissemination within soft tissues and the respiratory tract
- PMID: 20158613
- PMCID: PMC2904501
- DOI: 10.1111/j.1365-2958.2010.07065.x
Chemokine-cleaving Streptococcus pyogenes protease SpyCEP is necessary and sufficient for bacterial dissemination within soft tissues and the respiratory tract
Abstract
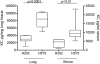
SpyCEP is a Streptococcus pyogenes protease that cleaves CXCL8/IL-8 and its activity is associated with human invasive disease severity. We investigated the role of SpyCEP in S. pyogenes necrotizing fasciitis and respiratory tract infection in mice using isogenic strains differing only in SpyCEP expression. SpyCEP cleaved human CXCL1, 2, 6 and 8 plus murine CXCL1 and 2 at a structurally conserved site. Mice were infected in thigh muscle with a strain of S. pyogenes that expresses a high level of SpyCEP, or with an isogenic non-SpyCEP expressing strain. SpyCEP expression by S. pyogenes hindered bacterial clearance from muscle, and enhanced bacterial spread, associated with cleavage of murine chemoattractant CXCL1. Mice were then infected with Lactococcus lactis strains that differed only in SpyCEP expression. In contrast to the parent L. lactis strain (lacks SpyCEP), which was avirulent when administered intramuscularly, infection with a strain that expressed SpyCEP heterologously led to dramatic systemic illness within 24 h, failure to clear bacteria from muscle and marked dissemination to other organs. In the upper airways, SpyCEP expression was required for survival of L. lactis but not S. pyogenes. However, dissemination of S. pyogenes to the lung was SpyCEP-dependent and was associated with evidence of chemokine cleavage. Taken together, the studies provide clear evidence that SpyCEP is necessary and sufficient for systemic bacterial dissemination from a soft tissue focus in this model and also underlies dissemination in the respiratory tract.
Figures






Similar articles
-
A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response.Infect Immun. 2008 Mar;76(3):978-85. doi: 10.1128/IAI.01354-07. Epub 2008 Jan 3. Infect Immun. 2008. PMID: 18174342 Free PMC article.
-
Impact of immunization against SpyCEP during invasive disease with two streptococcal species: Streptococcus pyogenes and Streptococcus equi.Vaccine. 2009 Aug 6;27(36):4923-9. doi: 10.1016/j.vaccine.2009.06.042. Epub 2009 Jun 27. Vaccine. 2009. PMID: 19563892 Free PMC article.
-
The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing.Cell Host Microbe. 2008 Aug 14;4(2):170-8. doi: 10.1016/j.chom.2008.07.002. Cell Host Microbe. 2008. PMID: 18692776 Free PMC article.
-
Molecular pathogenesis of necrotizing fasciitis.Annu Rev Pathol. 2010;5:1-31. doi: 10.1146/annurev-pathol-121808-102135. Annu Rev Pathol. 2010. PMID: 19737105 Review.
-
The Role of Streptococcal Cell-Envelope Proteases in Bacterial Evasion of the Innate Immune System.J Innate Immun. 2022;14(2):69-88. doi: 10.1159/000516956. Epub 2021 Oct 14. J Innate Immun. 2022. PMID: 34649250 Free PMC article. Review.
Cited by
-
Immunomodulating Enzymes from Streptococcus pyogenes-In Pathogenesis, as Biotechnological Tools, and as Biological Drugs.Microorganisms. 2024 Jan 18;12(1):200. doi: 10.3390/microorganisms12010200. Microorganisms. 2024. PMID: 38258026 Free PMC article. Review.
-
Impact of contusion injury on intramuscular emm1 group a streptococcus infection and lymphatic spread.Virulence. 2018;9(1):1074-1084. doi: 10.1080/21505594.2018.1482180. Virulence. 2018. PMID: 30052105 Free PMC article.
-
Pathogenicity Factors in Group C and G Streptococci.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.gpp3-0020-2018. doi: 10.1128/microbiolspec.GPP3-0020-2018. Microbiol Spectr. 2019. PMID: 31111818 Free PMC article. Review.
-
SpyA, a C3-like ADP-ribosyltransferase, contributes to virulence in a mouse subcutaneous model of Streptococcus pyogenes infection.Infect Immun. 2011 Jun;79(6):2404-11. doi: 10.1128/IAI.01191-10. Epub 2011 Mar 21. Infect Immun. 2011. PMID: 21422178 Free PMC article.
-
The CXC chemokine-degrading protease SpyCep of Streptococcus pyogenes promotes its uptake into endothelial cells.J Biol Chem. 2010 Sep 3;285(36):27798-805. doi: 10.1074/jbc.M109.098053. Epub 2010 Jun 18. J Biol Chem. 2010. PMID: 20562101 Free PMC article.
References
-
- Edwards RJ, Taylor GW, Ferguson M, Murray S, Rendell N, Wrigley A, et al. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J Infect Dis. 2005;192:783–790. - PubMed
-
- Engleberg NC, Heath A, Miller A, Rivera C, DiRita VJ. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J Infect Dis. 2001;183:1043–1054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

