Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution
- PMID: 20158900
- PMCID: PMC2829537
- DOI: 10.1186/1743-422X-7-38
Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution
Abstract
Background: Throughout the history of human influenza pandemics, pigs have been considered the most likely "mixing vessel" for reassortment between human and avian influenza viruses (AIVs). However, the replication efficiencies of influenza viruses from various hosts, as well as the expression of sialic acid (Sia) receptor variants in the entire porcine respiratory tract have never been studied in detail. Therefore, we established porcine nasal, tracheal, bronchial and lung explants, which cover the entire porcine respiratory tract with maximal similarity to the in vivo situation. Subsequently, we assessed virus yields of three porcine, two human and six AIVs in these explants. Since our results on virus replication were in disagreement with the previously reported presence of putative avian virus receptors in the trachea, we additionally studied the distribution of sialic acid receptors by means of lectin histochemistry. Human (Sia alpha2-6Gal) and avian virus receptors (Sia alpha2-3Gal) were identified with Sambucus Nigra and Maackia amurensis lectins respectively.
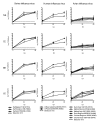
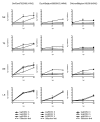
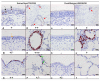
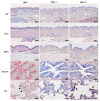
Results: Compared to swine and human influenza viruses, replication of the AIVs was limited in all cultures but most strikingly in nasal and tracheal explants. Results of virus titrations were confirmed by quantification of infected cells using immunohistochemistry. By lectin histochemistry we found moderate to abundant expression of the human-like virus receptors in all explant systems but minimal binding of the lectins that identify avian-like receptors, especially in the nasal, tracheal and bronchial epithelium.
Conclusions: The species barrier that restricts the transmission of influenza viruses from one host to another remains preserved in our porcine respiratory explants. Therefore this system offers a valuable alternative to study virus and/or host properties required for adaptation or reassortment of influenza viruses. Our results indicate that, based on the expression of Sia receptors alone, the pig is unlikely to be a more appropriate mixing vessel for influenza viruses than humans. We conclude that too little is known on the exact mechanism and on predisposing factors for reassortment to assess the true role of the pig in the emergence of novel influenza viruses.
Figures

Similar articles
-
The avian influenza A virus receptor SA-α2,3-Gal is expressed in the porcine nasal mucosa sustaining the pig as a mixing vessel for new influenza viruses.Virus Res. 2024 Feb;340:199304. doi: 10.1016/j.virusres.2023.199304. Epub 2024 Jan 3. Virus Res. 2024. PMID: 38142890 Free PMC article.
-
The infectivity of pandemic 2009 H1N1 and avian influenza viruses for pigs: an assessment by ex vivo respiratory tract organ culture.Influenza Other Respir Viruses. 2013 May;7(3):393-402. doi: 10.1111/j.1750-2659.2012.00397.x. Epub 2012 Jun 21. Influenza Other Respir Viruses. 2013. PMID: 22716314 Free PMC article.
-
Distribution of sialic acid receptors and influenza A virus of avian and swine origin in experimentally infected pigs.Virol J. 2011 Sep 8;8:434. doi: 10.1186/1743-422X-8-434. Virol J. 2011. PMID: 21902821 Free PMC article.
-
[Receptor sialylsugar chains as determinants of host range of influenza viruses].Nihon Rinsho. 2000 Nov;58(11):2206-10. Nihon Rinsho. 2000. PMID: 11225305 Review. Japanese.
-
[Swine influenza virus: evolution mechanism and epidemic characterization--a review].Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1138-45. Wei Sheng Wu Xue Bao. 2009. PMID: 20030049 Review. Chinese.
Cited by
-
Emergence of fatal avian influenza in New England harbor seals.mBio. 2012 Jul 31;3(4):e00166-12. doi: 10.1128/mBio.00166-12. Print 2012. mBio. 2012. PMID: 22851656 Free PMC article.
-
Swine Influenza Virus PA and Neuraminidase Gene Reassortment into Human H1N1 Influenza Virus Is Associated with an Altered Pathogenic Phenotype Linked to Increased MIP-2 Expression.J Virol. 2015 May;89(10):5651-67. doi: 10.1128/JVI.00087-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762737 Free PMC article.
-
The pig: a model for human infectious diseases.Trends Microbiol. 2012 Jan;20(1):50-7. doi: 10.1016/j.tim.2011.11.002. Epub 2011 Dec 5. Trends Microbiol. 2012. PMID: 22153753 Free PMC article. Review.
-
Glycan analysis and influenza A virus infection of primary swine respiratory epithelial cells: the importance of NeuAc{alpha}2-6 glycans.J Biol Chem. 2010 Oct 29;285(44):34016-26. doi: 10.1074/jbc.M110.115998. Epub 2010 Aug 19. J Biol Chem. 2010. PMID: 20724471 Free PMC article.
-
Establishment of sheep nasal mucosa explant model and its application in antiviral research.Front Microbiol. 2023 May 15;14:1124936. doi: 10.3389/fmicb.2023.1124936. eCollection 2023. Front Microbiol. 2023. PMID: 37256060 Free PMC article.
References
-
- Brown IH, Harris PA, McCauley JW, Alexander DJ. Multiple genetic reassortment of avian and human influenza A viruses in European pigs, resulting in the emergence of an H1N2 virus of novel genotype. J Gen Virol. 1998;79:2947–2955. - PubMed
-
- Olsen CW, Brown IH, Easterday BC, Van Reeth K. In: Diseases of Swine. 9. Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ, editor. Ames: Iowa State University Press; 2006. Swine Influenza; pp. 469–482.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

