Protein microarray: sensitive and effective immunodetection for drug residues
- PMID: 20158905
- PMCID: PMC2827365
- DOI: 10.1186/1472-6750-10-12
Protein microarray: sensitive and effective immunodetection for drug residues
Abstract
Background: Veterinary drugs such as clenbuterol (CL) and sulfamethazine (SM2) are low molecular weight (<1000 Da) compounds, or haptens, that are difficult to develop immunoassays due to their low immunogenicity. In this study, we conjugated the drugs to ovalbumin to increase their immunogenicity for antiserum production in rabbits and developed a protein microarray immunoassay for detection of clenbuterol and sulfamethazine. The sensitivity of this approach was then compared to traditional ELISA technique.
Results: The artificial antigens were spotted on microarray slides. Standard concentrations of the compounds were added to compete with the spotted antigens for binding to the antisera to determine the IC50. Our microarray assay showed the IC50 were 39.6 ng/ml for CL and 48.8 ng/ml for SM2, while the traditional competitive indirect-ELISA (ci-ELISA) showed the IC50 were 190.7 ng/ml for CL and 156.7 ng/ml for SM2. We further validated the two methods with CL fortified chicken muscle tissues, and the protein microarray assay showed 90% recovery while the ci-ELISA had 76% recovery rate. When tested with CL-fed chicken muscle tissues, the protein microarray assay had higher sensitivity (0.9 ng/g) than the ci-ELISA (0.1 ng/g) for detection of CL residues.
Conclusions: The protein microarrays showed 4.5 and 3.5 times lower IC50 than the ci-ELISA detection for CL and SM2, respectively, suggesting that immunodetection of small molecules with protein microarray is a better approach than the traditional ELISA technique.
Figures
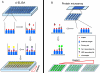
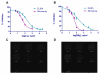
Similar articles
-
Application of quantum dot-antibody conjugates for detection of sulfamethazine residue in chicken muscle tissue.J Agric Food Chem. 2006 Aug 23;54(17):6139-42. doi: 10.1021/jf0606961. J Agric Food Chem. 2006. PMID: 16910698
-
Development of immunoassays for the detection of sulfamethazine in swine urine.Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2009 Mar;26(3):314-25. doi: 10.1080/02652030802520860. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2009. PMID: 19680904
-
Preparation of anti-salbutamol antibody based on a new designed immunogen and development of a heterologous indirect ELISA for detection of salbutamol residue.Yao Xue Xue Bao. 2010 Apr;45(4):442-50. Yao Xue Xue Bao. 2010. PMID: 21351723
-
Enzyme-linked immunoassay based on imprinted microspheres for the detection of sulfamethazine residue.J Chromatogr A. 2017 Jul 14;1506:9-17. doi: 10.1016/j.chroma.2017.05.016. Epub 2017 May 8. J Chromatogr A. 2017. PMID: 28545731
-
Small molecule microarrays for drug residue detection in foodstuffs.J Agric Food Chem. 2006 Sep 20;54(19):6978-83. doi: 10.1021/jf061105+. J Agric Food Chem. 2006. PMID: 16968051
Cited by
-
Specific plasma autoantibody reactivity in myelodysplastic syndromes.Sci Rep. 2013 Nov 22;3:3311. doi: 10.1038/srep03311. Sci Rep. 2013. PMID: 24264604 Free PMC article.
-
Protein Microarrays with Novel Microfluidic Methods: Current Advances.Microarrays (Basel). 2014 Jul 1;3(3):180-202. doi: 10.3390/microarrays3030180. Microarrays (Basel). 2014. PMID: 27600343 Free PMC article. Review.
-
The Simultaneous Detection of Multiple Antibiotics in Milk and Pork Based on an Antibody Chip Biosensor.Biosensors (Basel). 2022 Jul 29;12(8):578. doi: 10.3390/bios12080578. Biosensors (Basel). 2022. PMID: 36004974 Free PMC article.
-
Microarray technology for major chemical contaminants analysis in food: current status and prospects.Sensors (Basel). 2012;12(7):9234-52. doi: 10.3390/s120709234. Epub 2012 Jul 4. Sensors (Basel). 2012. PMID: 23012541 Free PMC article. Review.
References
-
- Paige JC, Tollefson L, Miller MA. Health implications of residues of veterinary drugs and chemicals in animal tissues. Vet Clin North Am Food Anim Pract. 1999;15:31–42. viii. - PubMed
-
- Toldrá F, Reig M. Methods for rapid detection of chemical and veterinary drug residues in animal foods. Trends in Food Science & Technology. 2006;17:482–489.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

