Identification of candidates for cyclotide biosynthesis and cyclisation by expressed sequence tag analysis of Oldenlandia affinis
- PMID: 20158917
- PMCID: PMC2838841
- DOI: 10.1186/1471-2164-11-111
Identification of candidates for cyclotide biosynthesis and cyclisation by expressed sequence tag analysis of Oldenlandia affinis
Abstract
Background: Cyclotides are a family of circular peptides that exhibit a range of biological activities, including anti-bacterial, cytotoxic, anti-HIV activities, and are proposed to function in plant defence. Their high stability has motivated their development as scaffolds for the stabilisation of peptide drugs. Oldenlandia affinis is a member of the Rubiaceae (coffee) family from which 18 cyclotides have been sequenced to date, but the details of their processing from precursor proteins have only begun to be elucidated. To increase the speed at which genes involved in cyclotide biosynthesis and processing are being discovered, an expressed sequence tag (EST) project was initiated to survey the transcript profile of O. affinis and to propose some future directions of research on in vivo protein cyclisation.
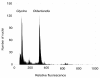
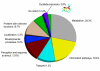
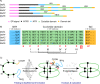
Results: Using flow cytometry the holoploid genome size (1C-value) of O. affinis was estimated to be 4,210 - 4,284 Mbp, one of the largest genomes of the Rubiaceae family. High-quality ESTs were identified, 1,117 in total, from leaf cDNAs and assembled into 502 contigs, comprising 202 consensus sequences and 300 singletons. ESTs encoding the cyclotide precursors for kalata B1 (Oak1) and kalata B2 (Oak4) were among the 20 most abundant ESTs. In total, 31 ESTs encoded cyclotide precursors, representing a distinct commitment of 2.8% of the O. affinis transcriptome to cyclotide biosynthesis. The high expression levels of cyclotide precursor transcripts are consistent with the abundance of mature cyclic peptides in O. affinis. A new cyclotide precursor named Oak5 was isolated and represents the first cDNA for the bracelet class of cyclotides in O. affinis. Clones encoding enzymes potentially involved in processing cyclotides were also identified and include enzymes involved in oxidative folding and proteolytic processing.
Conclusion: The EST library generated in this study provides a valuable resource for the study of the cyclisation of plant peptides. Further analysis of the candidates for cyclotide processing discovered in this work will increase our understanding and aid in reconstructing cyclotide production using transgenic systems and will benefit their development in pharmaceutical applications and insect-resistant crop plants.
Figures

References
-
- Gran L. An oxytocic principle found in Oldenlandia affinis DC. Medd Nor Farm Selsk. 1970;12:173–180.
-
- Sletten K, Gran L. Some molecular properties of kalatapeptide B-1. A uterotonic polypeptide isolated from Oldenlandia affinis DC. Medd Nor Farm Selsk. 1973;7-8:69–82.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

