High content screening of cortical neurons identifies novel regulators of axon growth
- PMID: 20159039
- PMCID: PMC2890283
- DOI: 10.1016/j.mcn.2010.02.002
High content screening of cortical neurons identifies novel regulators of axon growth
Abstract
Neurons in the central nervous system lose their intrinsic capacity for axon regeneration as they mature, and it is widely hypothesized that changes in gene expression are responsible. Testing this hypothesis and identifying the relevant genes has been challenging because hundreds to thousands of genes are developmentally regulated in CNS neurons, but only a small subset are likely relevant to axon growth. Here we used automated high content analysis (HCA) methods to functionally test 743 plasmids encoding developmentally regulated genes in neurite outgrowth assays using postnatal cortical neurons. We identified both growth inhibitors (Ephexin, Aldolase A, Solute Carrier 2A3, and Chimerin), and growth enhancers (Doublecortin, Doublecortin-like, Kruppel-like Factor 6, and CaM-Kinase II gamma), some of which regulate established growth mechanisms like microtubule dynamics and small GTPase signaling. Interestingly, with only one exception the growth-suppressing genes were developmentally upregulated, and the growth-enhancing genes downregulated. These data provide important support for the hypothesis that developmental changes in gene expression control neurite outgrowth, and identify potential new gene targets to promote neurite outgrowth.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
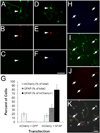


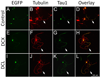
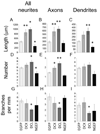

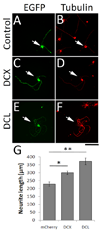
References
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. - PubMed
-
- Blackmore M, Letourneau PC. Changes within maturing neurons limit axonal regeneration in the developing spinal cord. J Neurobiol. 2006;66:348–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

