Bridging timescales and length scales: from macroscopic flux to the molecular mechanism of antibiotic diffusion through porins
- PMID: 20159153
- PMCID: PMC2820638
- DOI: 10.1016/j.bpj.2009.10.045
Bridging timescales and length scales: from macroscopic flux to the molecular mechanism of antibiotic diffusion through porins
Abstract
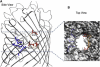
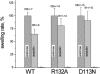
Our aim in this study was to provide an atomic description of ampicillin translocation through OmpF, the major outer membrane channel in Escherichia coli and main entry point for beta-lactam antibiotics. By applying metadynamics simulations, we also obtained the energy barriers along the diffusion pathway. We then studied the effect of mutations that affect the charge and size at the channel constriction zone, and found that in comparison to the wild-type, much lower energy barriers are required for translocation. The expected higher translocation rates were confirmed on the macroscopic scale by liposome-swelling assays. A microscopic view on the millisecond timescale was obtained by analysis of temperature-dependent ion current fluctuations in the presence of ampicillin and provide the enthalpic part of the energy barrier. By studying antibiotic translocation over various timescales and length scales, we were able to discern its molecular mechanism and rate-limiting interactions, and draw biologically relevant conclusions that may help in the design of drugs with enhanced permeation rates.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


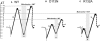
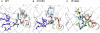

Similar articles
-
Millisecond-Long Simulations of Antibiotics Transport through Outer Membrane Channels.J Chem Theory Comput. 2021 Jan 12;17(1):549-559. doi: 10.1021/acs.jctc.0c01088. Epub 2020 Dec 30. J Chem Theory Comput. 2021. PMID: 33378186
-
Toward screening for antibiotics with enhanced permeation properties through bacterial porins.Biochemistry. 2010 Aug 17;49(32):6928-35. doi: 10.1021/bi100845x. Biochemistry. 2010. PMID: 20604536
-
Molecular simulations reveal the mechanism and the determinants for ampicillin translocation through OmpF.J Phys Chem B. 2010 Jul 29;114(29):9608-16. doi: 10.1021/jp9110579. J Phys Chem B. 2010. PMID: 20590090
-
Modified and Mutant Porins in the Study on Molecular Basis of Non- Specific Diffusion.Curr Protein Pept Sci. 2017;18(3):233-239. doi: 10.2174/1389203717666160905145514. Curr Protein Pept Sci. 2017. PMID: 27593088 Review.
-
Outer Membrane Porins.Subcell Biochem. 2019;92:79-123. doi: 10.1007/978-3-030-18768-2_4. Subcell Biochem. 2019. PMID: 31214985 Review.
Cited by
-
Altered antibiotic transport in OmpC mutants isolated from a series of clinical strains of multi-drug resistant E. coli.PLoS One. 2011;6(10):e25825. doi: 10.1371/journal.pone.0025825. Epub 2011 Oct 28. PLoS One. 2011. PMID: 22053181 Free PMC article.
-
Molecular Mechanism of Ciprofloxacin Translocation Through the Major Diffusion Channels of the ESKAPE Pathogens Klebsiella pneumoniae and Enterobacter cloacae.J Phys Chem B. 2024 Sep 5;128(35):8376-8387. doi: 10.1021/acs.jpcb.4c03327. Epub 2024 Aug 23. J Phys Chem B. 2024. PMID: 39180156 Free PMC article.
-
Conformational flexibility driving charge-selective substrate translocation across a bacterial transporter.Chem Sci. 2024 May 13;15(24):9333-9344. doi: 10.1039/d4sc00345d. eCollection 2024 Jun 19. Chem Sci. 2024. PMID: 38903220 Free PMC article.
-
Understanding antibiotic resistance via outer membrane permeability.Infect Drug Resist. 2018 Apr 11;11:523-530. doi: 10.2147/IDR.S156995. eCollection 2018. Infect Drug Resist. 2018. PMID: 29695921 Free PMC article. Review.
-
Implication of porins in beta-lactam resistance of Providencia stuartii.J Biol Chem. 2010 Oct 15;285(42):32273-81. doi: 10.1074/jbc.M110.143305. Epub 2010 Jul 28. J Biol Chem. 2010. PMID: 20667831 Free PMC article.
References
-
- Arias C.A., Murray B.E. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N. Engl. J. Med. 2009;360:439–443. - PubMed
-
- Spellberg B., Powers J.H., Edwards J.E. Trends in antimicrobial drug development: implications for the future. Clin. Infect. Dis. 2004;38:1279–1286. - PubMed
-
- Barker J.J. Antibacterial drug discovery and structure-based design. Drug Discov. Today. 2006;11:391–404. - PubMed
-
- Pagès J.M., James C.E., Winterhalter M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008;6:893–903. - PubMed
-
- Cowan S.W., Schirmer T., Rosenbusch J.P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

