Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis
- PMID: 20159257
- PMCID: PMC4259249
- DOI: 10.1016/j.jaci.2009.09.054
Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis
Abstract
Background: The cytokine IL-9 has been implicated in allergic reactions, including food allergy, but its contribution to parenteral versus oral antigen-induced anaphylaxis remains unclear.
Objectives: We sought to delineate the contribution of the IL-9/IL-9 receptor alpha-chain (IL-9R) pathway to parenteral and oral antigen-induced anaphylaxis.
Methods: Wild-type, IL-9-deficient (Il9(-/-)), and IL-9R-deficient (Il9R(-/-)) mice were subjected to passive and active parenteral and oral antigen (ovalbumin [OVA])-induced anaphylaxis. Severity of systemic anaphylaxis was gauged by decreased body temperature; intestinal anaphylaxis was assessed based on secretory diarrhea, intestinal mastocytosis, and serum murine mast cell protease 1 level. Specific immunoglobulin isotypes or immunoglobulin receptor-blocking antibodies were administered before challenge to define the role of the IgE and IgG pathways.
Results: Repeated oral antigen challenge of OVA-sensitized wild-type mice induced anaphylaxis with both systemic and intestinal involvement; both were IgE dependent and attenuated in Il9(-/-) and Il9R(-/-) mice. In contrast, parenteral OVA challenge of OVA-sensitized wild-type mice induced systemic anaphylaxis, which was independent of the IL-9/IL-9R pathway. Strikingly, the IL-9/IL-9R pathway had no role in either the IgG or IgE component of parenteral antigen-induced or anti-IgE and anti-FcgammaRII/III mAb-induced systemic anaphylaxis.
Conclusions: Parenteral antigen-induced murine systemic anaphylaxis is mediated by both IgG- and IgE-dependent pathways, and both can occur independently of IL-9/IL-9R signaling. In contrast, oral antigen-induced intestinal and systemic anaphylaxis is strictly IgE mediated and requires IL-9/IL-9R signaling. These studies indicate differential involvement of the IL-9/IL-9R pathway in systemic and oral antigen-induced anaphylaxis.
Copyright 2010 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: F. D. Finkelman is a consultant for Abbott and Associate Editor for the Journal of Allergy and Clinical Immunology, has received research support from Abbott, and is treasurer-elect for the American Association of Immunologists. The rest of the authors have declared that they have no conflict of interest.
Figures
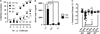
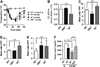
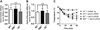
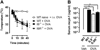

Similar articles
-
Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis.J Allergy Clin Immunol. 2013 Feb;131(2):451-60.e1-6. doi: 10.1016/j.jaci.2012.11.032. J Allergy Clin Immunol. 2013. PMID: 23374269 Free PMC article.
-
IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling.J Allergy Clin Immunol. 2011 Mar;127(3):795-805.e1-6. doi: 10.1016/j.jaci.2010.11.009. Epub 2010 Dec 17. J Allergy Clin Immunol. 2011. PMID: 21167580 Free PMC article.
-
IL-9 promotes but is not necessary for systemic anaphylaxis.J Immunol. 2005 Jul 1;175(1):335-41. doi: 10.4049/jimmunol.175.1.335. J Immunol. 2005. PMID: 15972666
-
Anaphylaxis: lessons from mouse models.J Allergy Clin Immunol. 2007 Sep;120(3):506-15; quiz 516-7. doi: 10.1016/j.jaci.2007.07.033. J Allergy Clin Immunol. 2007. PMID: 17765751 Review.
-
Molecular mechanisms of anaphylaxis: lessons from studies with murine models.J Allergy Clin Immunol. 2005 Mar;115(3):449-57; quiz 458. doi: 10.1016/j.jaci.2004.12.1125. J Allergy Clin Immunol. 2005. PMID: 15753886 Review.
Cited by
-
Discovery of an agonistic Siglec-6 antibody that inhibits and reduces human mast cells.Commun Biol. 2022 Nov 11;5(1):1226. doi: 10.1038/s42003-022-04207-w. Commun Biol. 2022. PMID: 36369358 Free PMC article.
-
Role of IL-9 and STATs in hematological malignancies (Review).Oncol Lett. 2014 Mar;7(3):602-610. doi: 10.3892/ol.2013.1761. Epub 2013 Dec 16. Oncol Lett. 2014. PMID: 24520283 Free PMC article.
-
Elevated antigen-driven IL-9 responses are prominent in peanut allergic humans.PLoS One. 2012;7(10):e45377. doi: 10.1371/journal.pone.0045377. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071516 Free PMC article.
-
IL-4 receptor alpha signaling alters oral food challenge and immunotherapy outcomes in mice.J Allergy Clin Immunol. 2023 Jan;151(1):182-191.e6. doi: 10.1016/j.jaci.2022.07.011. Epub 2022 Aug 4. J Allergy Clin Immunol. 2023. PMID: 35934083 Free PMC article.
-
Increased susceptibility of 129SvEvBrd mice to IgE-Mast cell mediated anaphylaxis.BMC Immunol. 2011 Feb 3;12:14. doi: 10.1186/1471-2172-12-14. BMC Immunol. 2011. PMID: 21291538 Free PMC article.
References
-
- Simons FE, Frew AJ, Ansotegui IJ, Bochner BS, Golden DB, Finkelman FD, et al. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;120(suppl):S2–S4. - PubMed
-
- Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hyper-sensitivity reactions to foods, drugs, and insects. J Allergy Clin Immunol. 2005;116:153–163. - PubMed
-
- Greenberger PA. Drug Allergy. J Allergy Clin Immunol. 2006;117(suppl):S464–S470. - PubMed
-
- Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. - PubMed
-
- Wang J, Sampson HA. Food anaphylaxis. Clin Exp Allergy. 2007;37:651–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

