Azidothymidine enhances fluorodeoxyuridine-mediated radiosensitization
- PMID: 20159365
- PMCID: PMC2900777
- DOI: 10.1016/j.ijrobp.2009.09.016
Azidothymidine enhances fluorodeoxyuridine-mediated radiosensitization
Abstract
Purpose: To examine the role of DNA repair and altered thymidine analogues in altering the response to radiation during thymidine deprivation.
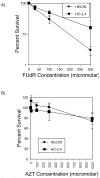
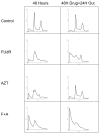
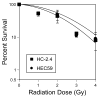
Methods and materials: Mismatch repair-deficient and -proficient cell lines HEC59 and HC-2.4 were treated with fluorodeoxyuridine (FUdR), azidothymidine (AZT), and irradiation either alone or in combination, and outcomes of clonogenic survival and cell-cycle distributions were determined.
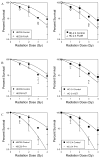
Results: Survival outcomes for all treatments were similar for both cell lines, suggesting that hMSH2 does not significantly influence thymidine deprivation toxicity or radiosensitization. The chain-terminating thymidine analogue AZT increased the toxicity of FUdR and increased DNA fragmentation. The combination of FUdR and AZT afforded greater radiosensitization than either drug alone. Drug enhancement ratios, the degree of excess radiation-induced cell death in drug-treated cultures compared with radiation alone for HEC59, were 1.2, 1.4, and 1.8 for AZT, FUdR, and the combination, respectively. Enhancement ratios for HC-2.4 were 1.3, 1.5, and 1.8 for AZT, FUdR, and the combination, respectively.
Conclusion: Azidothymidine, a chain-terminating thymidine analogue, can enhance the radiosensitizing affects of thymidine deprivation. Deoxyribonucleic acid strand breaks may play an important role in the mechanism of thymidine deprivation-induced radiosensitization.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Notification
The authors have no conflicts to report.
Figures

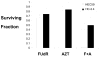
Similar articles
-
Selective radiosensitization of drug-resistant MutS homologue-2 (MSH2) mismatch repair-deficient cells by halogenated thymidine (dThd) analogues: Msh2 mediates dThd analogue DNA levels and the differential cytotoxicity and cell cycle effects of the dThd analogues and 6-thioguanine.Cancer Res. 2000 Oct 15;60(20):5773-80. Cancer Res. 2000. PMID: 11059773
-
Fluorodeoxyuridine-mediated modulation of iododeoxyuridine incorporation and radiosensitization in human colon cancer cells in vitro and in vivo.Cancer Res. 1991 Aug 1;51(15):3900-5. Cancer Res. 1991. PMID: 1830239
-
Fluorodeoxyuridine-induced radiosensitization and inhibition of DNA double strand break repair in human colon cancer cells.Int J Radiat Oncol Biol Phys. 1990 Dec;19(6):1411-7. doi: 10.1016/0360-3016(90)90352-k. Int J Radiat Oncol Biol Phys. 1990. PMID: 2148170
-
Short hairpin RNA suppression of thymidylate synthase produces DNA mismatches and results in excellent radiosensitization.Int J Radiat Oncol Biol Phys. 2012 Dec 1;84(5):e613-20. doi: 10.1016/j.ijrobp.2012.06.050. Epub 2012 Aug 3. Int J Radiat Oncol Biol Phys. 2012. PMID: 22867891 Free PMC article.
-
The role of cell cycle redistribution in radiosensitization: implications regarding the mechanism of fluorodeoxyuridine radiosensitization.Int J Radiat Oncol Biol Phys. 1994 Nov 15;30(4):851-9. doi: 10.1016/0360-3016(94)90360-3. Int J Radiat Oncol Biol Phys. 1994. PMID: 7960987
References
-
- Ingraham HA, Dickey L, Goulian M. DNA fragmentation and cytotoxicity from increased cellular deoxyuridylate. Biochemistry. 1986;25:3225–3230. - PubMed
-
- Radany ER, Dornfeld KJ. Distinct Roles of Uracil-DNA Base Excision Repair in Fluoropyrimidine-Mediated Radiosensitization and Direct Cytotoxicity in Human Tumor Cells. International Journal of Radiation Oncology, Biology and Physics. 2005 October 1;63:S482–S483.
-
- Allen B, Johnson M, Marsh A, Dornfeld KJ. Base excision repair of both uracil and oxidatively damaged bases contribute to thymidine deprivation induced radiosensitization. International Journal of Radiation Oncology, Biology, Physics. 2006;65:1544–1552. - PubMed
-
- Perillo-Adamer F, Delaloye AB, Genton CS, Schaffland AO, Dupertuis YM, Buchegger F. Short fluorodeoxyuridine exposure of different human glioblastoma lines induces high-level accumulation of S-phase cells that avidly incorporate 125I-iododeoxyuridine. Eur J Nucl Med Mol Imaging. 2006 May;33(5):613–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

