Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice
- PMID: 20159452
- PMCID: PMC6366558
- DOI: 10.1016/j.neuron.2010.01.009
Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice
Abstract
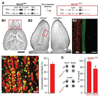
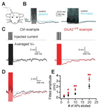
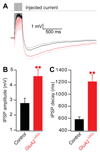
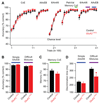
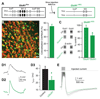
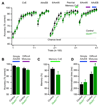
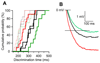
Local inhibitory circuits are thought to shape neuronal information processing in the central nervous system, but it remains unclear how specific properties of inhibitory neuronal interactions translate into behavioral performance. In the olfactory bulb, inhibition of mitral/tufted cells via granule cells may contribute to odor discrimination behavior by refining neuronal representations of odors. Here we show that selective deletion of the AMPA receptor subunit GluA2 in granule cells boosted synaptic Ca(2+) influx, increasing inhibition of mitral cells. On a behavioral level, discrimination of similar odor mixtures was accelerated while leaving learning and memory unaffected. In contrast, selective removal of NMDA receptors in granule cells slowed discrimination of similar odors. Our results demonstrate that inhibition of mitral cells controlled by granule cell glutamate receptors results in fast and accurate discrimination of similar odors. Thus, spatiotemporally defined molecular perturbations of olfactory bulb granule cells directly link stimulus similarity, neuronal processing time, and discrimination behavior to synaptic inhibition.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Linking local circuit inhibition to olfactory behavior: a critical role for granule cells in olfactory discrimination.Neuron. 2010 Feb 11;65(3):295-7. doi: 10.1016/j.neuron.2010.01.029. Neuron. 2010. PMID: 20159443
References
-
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. - PubMed
-
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. - PubMed
-
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. - PubMed
-
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

