Structure of the Saccharomyces cerevisiae Cet1-Ceg1 mRNA capping apparatus
- PMID: 20159466
- PMCID: PMC2877398
- DOI: 10.1016/j.str.2009.12.009
Structure of the Saccharomyces cerevisiae Cet1-Ceg1 mRNA capping apparatus
Abstract
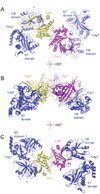
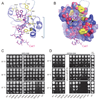
The 5' guanine-N7 cap is the first cotranscriptional modification of messenger RNA. In Saccharomyces cerevisiae, the first two steps in capping are catalyzed by the RNA triphosphatase Cet1 and RNA guanylyltransferase Ceg1, which form a complex that is directly recruited to phosphorylated RNA polymerase II (RNAP IIo), primarily via contacts between RNAP IIo and Ceg1. A 3.0 A crystal structure of Cet1-Ceg1 revealed a 176 kDa heterotetrameric complex composed of one Cet1 homodimer that associates with two Ceg1 molecules via interactions between the Ceg1 oligonucleotide binding domain and an extended Cet1 WAQKW amino acid motif. The WAQKW motif is followed by a flexible linker that would allow Ceg1 to achieve conformational changes required for capping while maintaining interactions with both Cet1 and RNAP IIo. The impact of mutations as assessed through genetic analysis in S. cerevisiae is consonant with contacts observed in the Cet1-Ceg1 structure.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
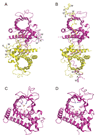




Similar articles
-
Genetic, physical, and functional interactions between the triphosphatase and guanylyltransferase components of the yeast mRNA capping apparatus.Mol Cell Biol. 1998 Sep;18(9):5189-98. doi: 10.1128/MCB.18.9.5189. Mol Cell Biol. 1998. PMID: 9710603 Free PMC article.
-
The essential interaction between yeast mRNA capping enzyme subunits is not required for triphosphatase function in vivo.Mol Cell Biol. 2000 Dec;20(24):9307-16. doi: 10.1128/MCB.20.24.9307-9316.2000. Mol Cell Biol. 2000. PMID: 11094081 Free PMC article.
-
An essential function of Saccharomyces cerevisiae RNA triphosphatase Cet1 is to stabilize RNA guanylyltransferase Ceg1 against thermal inactivation.J Biol Chem. 2001 Sep 28;276(39):36116-24. doi: 10.1074/jbc.M105856200. Epub 2001 Jul 19. J Biol Chem. 2001. PMID: 11463793
-
Enzymology of RNA cap synthesis.Wiley Interdiscip Rev RNA. 2010 Jul-Aug;1(1):152-72. doi: 10.1002/wrna.19. Epub 2010 May 25. Wiley Interdiscip Rev RNA. 2010. PMID: 21956912 Free PMC article. Review.
-
The mRNA capping apparatus as drug target and guide to eukaryotic phylogeny.Cold Spring Harb Symp Quant Biol. 2001;66:301-12. doi: 10.1101/sqb.2001.66.301. Cold Spring Harb Symp Quant Biol. 2001. PMID: 12762032 Review. No abstract available.
Cited by
-
Cross-talk of phosphorylation and prolyl isomerization of the C-terminal domain of RNA Polymerase II.Molecules. 2014 Jan 27;19(2):1481-511. doi: 10.3390/molecules19021481. Molecules. 2014. PMID: 24473209 Free PMC article. Review.
-
How an mRNA capping enzyme reads distinct RNA polymerase II and Spt5 CTD phosphorylation codes.Genes Dev. 2014 Jun 15;28(12):1323-36. doi: 10.1101/gad.242768.114. Genes Dev. 2014. PMID: 24939935 Free PMC article.
-
RNAP II produces capped 18S and 25S ribosomal RNAs resistant to 5'-monophosphate dependent processive 5' to 3' exonuclease in polymerase switched Saccharomyces cerevisiae.BMC Mol Cell Biol. 2022 Apr 10;23(1):17. doi: 10.1186/s12860-022-00417-6. BMC Mol Cell Biol. 2022. PMID: 35399070 Free PMC article.
-
The conserved foot domain of RNA pol II associates with proteins involved in transcriptional initiation and/or early elongation.Genetics. 2011 Dec;189(4):1235-48. doi: 10.1534/genetics.111.133215. Epub 2011 Sep 27. Genetics. 2011. PMID: 21954159 Free PMC article.
-
Crystal structure of vaccinia virus mRNA capping enzyme provides insights into the mechanism and evolution of the capping apparatus.Structure. 2014 Mar 4;22(3):452-65. doi: 10.1016/j.str.2013.12.014. Structure. 2014. PMID: 24607143 Free PMC article.
References
-
- Benarroch D, Smith P, Shuman S. Characterization of a trifunctional mimivirus mRNA capping enzyme and crystal structure of the triphosphatase domain. Structure. 2008;16:501–512. - PubMed
-
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta. Crystallogr. D Biol. Crystallogr. 1998;54:905–921. - PubMed
-
- Chiu YL, Ho CK, Saha N, Schwer B, Shuman S, Rana TM. Tat stimulates cotranscriptional capping of HIV mRNA. Mol. Cell. 2002;10:585–597. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

