Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish
- PMID: 20159593
- PMCID: PMC2824622
- DOI: 10.1016/j.devcel.2009.11.015
Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish
Erratum in
- Dev Cell. 2011 Aug 16;21(2):384
Abstract
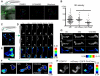
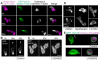
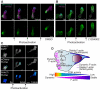
Cell polarity is crucial for directed migration. Here we show that phosphoinositide 3-kinase (PI(3)K) mediates neutrophil migration in vivo by differentially regulating cell protrusion and polarity. The dynamics of PI(3)K products PI(3,4,5)P(3)-PI(3,4)P(2) during neutrophil migration were visualized in living zebrafish, revealing that PI(3)K activation at the leading edge is critical for neutrophil motility in intact tissues. A genetically encoded photoactivatable Rac was used to demonstrate that localized activation of Rac is sufficient to direct migration with precise temporal and spatial control in vivo. Similar stimulation of PI(3)K-inhibited cells did not direct migration. Localized Rac activation rescued membrane protrusion but not anteroposterior polarization of F-actin dynamics of PI(3)K-inhibited cells. Uncoupling Rac-mediated protrusion and polarization suggests a paradigm of two-tiered PI(3)K-mediated regulation of cell motility. This work provides new insight into how cell signaling at the front and back of the cell is coordinated during polarized cell migration in intact tissues within a multicellular organism.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures

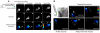

Comment in
-
Sensing and controlling protein dynamics.Nat Rev Mol Cell Biol. 2010 Oct;11(10):680-1. doi: 10.1038/nrm2985. Nat Rev Mol Cell Biol. 2010. PMID: 20861876 No abstract available.
-
Guided by the light: neutrophil migration through zebrafish.Dev Cell. 2011 Mar 15;20(3):e2. doi: 10.1016/j.devcel.2011.03.005. Dev Cell. 2011. PMID: 21411394 No abstract available.
References
-
- Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. - PubMed
-
- Arrieumerlou C, Meyer T. A local coupling model and compass parameter for eukaryotic chemotaxis. Dev Cell. 2005;8:215–227. - PubMed
-
- Barberis L, Hirsch E. Targeting phosphoinositide 3-kinase gamma to fight inflammation and more. Thromb Haemost. 2008;99:279–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

