A DNA damage-activated checkpoint kinase phosphorylates tau and enhances tau-induced neurodegeneration
- PMID: 20159774
- PMCID: PMC2860892
- DOI: 10.1093/hmg/ddq068
A DNA damage-activated checkpoint kinase phosphorylates tau and enhances tau-induced neurodegeneration
Abstract
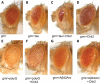
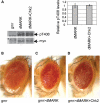
Hyperphosphorylation of the microtubule associated protein tau is detected in the brains of individuals with a range of neurodegenerative diseases including Alzheimer's disease (AD). An imbalance in phosphorylation and/or dephosphorylation of tau at disease-related sites has been suggested to initiate the abnormal metabolism and toxicity of tau in disease pathogenesis. However, the mechanisms underlying abnormal phosphorylation of tau in AD are not fully understood. Here, we show that the DNA damage-activated Checkpoint kinase 2 (Chk2) is a novel tau kinase and enhances tau toxicity in a transgenic Drosophila model. Overexpression of Drosophila Chk2 increases tau phosphorylation at Ser262 and enhances tau-induced neurodegeneration in transgenic flies expressing human tau. The non-phosphorylatable Ser262Ala mutation abolishes Chk2-induced enhancement of tau toxicity, suggesting that the Ser262 phosphorylation site is involved in the enhancement of tau toxicity by Chk2. In vitro kinase assays revealed that human Chk2 and a closely related checkpoint kinase 1 (Chk1) directly phosphorylate human tau at Ser262. We also demonstrate that Drosophila Chk2 does not modulate the activity of the fly homolog of microtubule affinity regulating kinase, which has been shown to be a physiological tau Ser262 kinase. Since accumulation of DNA damage has been detected in the brains of AD patients, our results suggest that the DNA damage-activated kinases Chk1 and Chk2 may be involved in tau phosphorylation and toxicity in the pathogenesis of AD.
Figures



Similar articles
-
Tau Ser262 phosphorylation is critical for Abeta42-induced tau toxicity in a transgenic Drosophila model of Alzheimer's disease.Hum Mol Genet. 2010 Aug 1;19(15):2947-57. doi: 10.1093/hmg/ddq200. Epub 2010 May 12. Hum Mol Genet. 2010. PMID: 20466736 Free PMC article.
-
Global analysis of phosphorylation of tau by the checkpoint kinases Chk1 and Chk2 in vitro.J Proteome Res. 2013 Jun 7;12(6):2654-65. doi: 10.1021/pr400008f. Epub 2013 Apr 26. J Proteome Res. 2013. PMID: 23550703 Free PMC article.
-
Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer's disease-related tau phosphorylation via PAR-1.PLoS Genet. 2012;8(8):e1002918. doi: 10.1371/journal.pgen.1002918. Epub 2012 Aug 30. PLoS Genet. 2012. PMID: 22952452 Free PMC article.
-
Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2.Curr Opin Cell Biol. 2009 Apr;21(2):245-55. doi: 10.1016/j.ceb.2009.01.018. Epub 2009 Feb 21. Curr Opin Cell Biol. 2009. PMID: 19230643 Free PMC article. Review.
-
Taking the time to make important decisions: the checkpoint effector kinases Chk1 and Chk2 and the DNA damage response.DNA Repair (Amst). 2009 Sep 2;8(9):1047-54. doi: 10.1016/j.dnarep.2009.04.012. Epub 2009 May 26. DNA Repair (Amst). 2009. PMID: 19473886 Free PMC article. Review.
Cited by
-
Tau hyperphosphorylation is associated with spatial learning and memory after exposure to benzo[a]pyrene in SD rats.Neurotox Res. 2013 Nov;24(4):461-71. doi: 10.1007/s12640-013-9387-2. Epub 2013 Mar 19. Neurotox Res. 2013. PMID: 23508864
-
Drosophila models of tauopathies: what have we learned?Int J Alzheimers Dis. 2012;2012:970980. doi: 10.1155/2012/970980. Epub 2012 Jun 4. Int J Alzheimers Dis. 2012. PMID: 22701808 Free PMC article.
-
Evaluation of traditional medicines for neurodegenerative diseases using Drosophila models.Evid Based Complement Alternat Med. 2014;2014:967462. doi: 10.1155/2014/967462. Epub 2014 Mar 26. Evid Based Complement Alternat Med. 2014. PMID: 24790636 Free PMC article. Review.
-
Environmental factors in the development and progression of late-onset Alzheimer's disease.Neurosci Bull. 2014 Apr;30(2):253-70. doi: 10.1007/s12264-013-1425-9. Epub 2014 Mar 24. Neurosci Bull. 2014. PMID: 24664867 Free PMC article. Review.
-
Emerging Evidences for an Implication of the Neurodegeneration-Associated Protein TAU in Cancer.Brain Sci. 2020 Nov 16;10(11):862. doi: 10.3390/brainsci10110862. Brain Sci. 2020. PMID: 33207722 Free PMC article. Review.
References
-
- Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. - PubMed
-
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y.C., Zaidi M.S., Wisniewski H.M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 1986;261:6084–6089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

