Thoracic aortic disease in tuberous sclerosis complex: molecular pathogenesis and potential therapies in Tsc2+/- mice
- PMID: 20159776
- PMCID: PMC2860890
- DOI: 10.1093/hmg/ddq066
Thoracic aortic disease in tuberous sclerosis complex: molecular pathogenesis and potential therapies in Tsc2+/- mice
Abstract
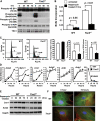
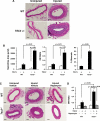
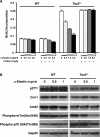
Tuberous sclerosis complex (TSC) is a genetic disorder with pleiotropic manifestations caused by heterozygous mutations in either TSC1 or TSC2. One of the less investigated complications of TSC is the formation of aneurysms of the descending aorta, which are characterized on pathologic examination by smooth muscle cell (SMC) proliferation in the aortic media. SMCs were explanted from Tsc2(+/-) mice to investigate the pathogenesis of aortic aneurysms caused by TSC2 mutations. Tsc2(+/-) SMCs demonstrated increased phosphorylation of mammalian target of rapamycin (mTOR), S6 and p70S6K and increased proliferation rates compared with wild-type (WT) SMCs. Tsc2(+/-) SMCs also had reduced expression of SMC contractile proteins compared with WT SMCs. An inhibitor of mTOR signaling, rapamycin, decreased SMC proliferation and increased contractile protein expression in the Tsc2(+/-) SMCs to levels similar to WT SMCs. Exposure to alpha-elastin fragments also decreased proliferation of Tsc2(+/-) SMCs and increased levels of p27(kip1), but failed to increase expression of contractile proteins. In response to artery injury using a carotid artery ligation model, Tsc2(+/-) mice significantly increased neointima formation compared with the control mice, and the neointima formation was inhibited by treatment with rapamycin. These results demonstrate that Tsc2 haploinsufficiency in SMCs increases proliferation and decreases contractile protein expression and suggest that the increased proliferative potential of the mutant cells may be suppressed in vivo by interaction with elastin. These findings provide insights into the molecular pathogenesis of aortic disease in TSC patients and identify a potential therapeutic target for treatment of this complication of the disease.
Figures


References
-
- Osborne J.P., Fryer A., Webb D. Epidemiology of tuberous sclerosis. Ann. N. Y. Acad. Sci. 1991;615:125–127. - PubMed
-
- van Slegtenhorst M., de Hoogt R., Hermans C., Nellist M., Janssen B., Verhoef S., Lindhout D., van den O.A., Halley D., Young J., et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. - PubMed
-
- The European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. - PubMed
-
- Wienecke R., Konig A., DeClue J.E. Identification of tuberin, the tuberous sclerosis-2 product. Tuberin possesses specific Rap1GAP activity. J. Biol. Chem. 1995;270:16409–16414. - PubMed
-
- Xiao G.H., Shoarinejad F., Jin F., Golemis E.A., Yeung R.S. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J. Biol. Chem. 1997;272:6097–6100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

