Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer
- PMID: 20159814
- PMCID: PMC3040042
- DOI: 10.1200/JCO.2009.25.9259
Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer
Abstract
Purpose: Persistence of ligand-mediated androgen receptor signaling has been documented in castration-resistant prostate cancers (CRPCs). Abiraterone acetate (AA) is a potent and selective inhibitor of CYP17, which is required for androgen biosynthesis in the testes, adrenal glands, and prostate tissue. This trial evaluated the efficacy and safety of AA in combination with prednisone to reduce the symptoms of secondary hyperaldosteronism that can occur with AA monotherapy.
Patients and methods: Fifty-eight men with progressive metastatic CRPC who experienced treatment failure with docetaxel-based chemotherapy received AA (1,000 mg daily) with prednisone (5 mg twice daily). Twenty-seven (47%) patients had received prior ketoconazole. The primary outcome was > or = 50% prostate-specific antigen (PSA) decline, with objective response by Response Evaluation Criteria in Solid Tumors (RECIST) criteria, and changes in Eastern Cooperative Oncology Group (ECOG) performance status (PS) and circulating tumor cell (CTC) numbers. Safety was also evaluated.
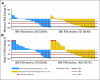
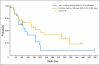
Results: A > or = 50% decline in PSA was confirmed in 22 (36%) patients, including 14 (45%) of 31 ketoconazole-naïve and seven (26%) of 27 ketoconazole-pretreated patients. Partial responses were seen in four (18%) of 22 patients with RECIST-evaluable target lesions. Improved ECOG PS was seen in 28% of patients. Median time to PSA progression was 169 days (95% CI, 82 to 200 days). CTC conversions with treatment from > or = 5 to < 5 were noted in 10 (34%) of 29 patients. The majority of AA-related adverse events were grade 1 to 2, and no AA-related grade 4 events were seen.
Conclusion: AA plus prednisone was well tolerated, with encouraging antitumor activity in heavily pretreated CRPC patients. The incidence of mineralocorticoid-related toxicities (hypertension or hypokalemia) was reduced by adding low-dose prednisone. The combination of AA plus prednisone is recommended for phase III investigations.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Castration-resistant prostate cancer--hormone therapy redux.J Clin Oncol. 2010 Mar 20;28(9):1447-9. doi: 10.1200/JCO.2009.25.3781. Epub 2010 Feb 16. J Clin Oncol. 2010. PMID: 20159817 No abstract available.
References
-
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. - PubMed
-
- Geller J, Albert JD, Nochstein DA, et al. Comparison of prostatic cancer tissue dehydrotestosterone levels at the time of relapse following orchiectomy or estrogen therapy. J Urol. 1984;132:693–696. - PubMed
-
- Suzuki K, Nishiyama T, Hara N, et al. Importance of the intracrine metabolism of adrenal androgens in androgen-dependent prostate cancer. Prostate Cancer Prostatic Dis. 2007;10:301–306. - PubMed
-
- Titus MA, Schell MJ, Lih FB, et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. - PubMed
-
- Nelson PS, Han D, Rochon Y, et al. Comprehensive analyses of prostate gene expression: Convergence of expressed sequence tag databases, transcript profiling and proteomics. Electrophoresis. 2000;21:11–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

