Neoadjuvant clinical trial with sorafenib for patients with stage II or higher renal cell carcinoma
- PMID: 20159822
- PMCID: PMC4525128
- DOI: 10.1200/JCO.2009.24.7759
Neoadjuvant clinical trial with sorafenib for patients with stage II or higher renal cell carcinoma
Abstract
Purpose: The multitargeted tyrosine kinase inhibitor sorafenib is used for the treatment of advanced-stage renal cell carcinoma. However, the safety and efficacy of this agent have yet to be evaluated in the preoperative period, where there may be potential advantages including tumor downstaging. This prospective trial evaluates the safety and feasibility of sorafenib in the preoperative setting.
Patients and methods: Thirty patients with clinical stage II or higher renal masses, selected based on their candidacy for nephrectomy, underwent preoperative treatment with sorafenib. Toxicities, surgical complications, and tumor responses were monitored.
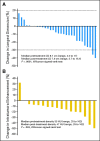
Results: Of the thirty patients enrolled, 17 patients had localized disease and 13 had metastatic disease. After a course of sorafenib therapy (median duration, 33 days), a decrease in primary tumor size (median, 9.6%) and radiographic evidence of loss of intratumoral enhancement, quantified using a methodology similar to Choi criteria (median, 13%), was also observed. According to Response Evaluation Criteria in Solid Tumors, of the 28 patients evaluable for response, two patients had a partial response and 26 had stable disease, with no patients progressing on therapy. Toxicities from sorafenib were similar to that expected with this class of medication. All patients were able to proceed with nephrectomy and no surgical complications related to sorafenib administration were observed.
Conclusion: The administration of preoperative sorafenib therapy can impact the size and density of the primary tumor and appears safe and feasible. Further studies are required to determine if preoperative systemic therapy improves outcomes in patients undergoing nephrectomy for renal cell carcinoma.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Frequent dose interruptions are required for patients receiving oral kinase inhibitor therapy for advanced renal cell carcinoma.Am J Clin Oncol. 2010 Jun;33(3):217-20. doi: 10.1097/COC.0b013e3181a650a6. Am J Clin Oncol. 2010. PMID: 19745694
-
Sequential use of the tyrosine kinase inhibitors sorafenib and sunitinib in metastatic renal cell carcinoma: a retrospective outcome analysis.Eur Urol. 2008 Dec;54(6):1373-8. doi: 10.1016/j.eururo.2008.07.051. Epub 2008 Aug 8. Eur Urol. 2008. PMID: 18692304
-
Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib.Br J Dermatol. 2009 Nov;161(5):1045-51. doi: 10.1111/j.1365-2133.2009.09290.x. Epub 2009 May 5. Br J Dermatol. 2009. PMID: 19558553
-
Sorafenib: recent update on activity as a single agent and in combination with interferon-alpha2 in patients with advanced-stage renal cell carcinoma.Clin Genitourin Cancer. 2006 Mar;4(4):246-8. doi: 10.3816/cgc.2006.n.002. Clin Genitourin Cancer. 2006. PMID: 16729906 Review.
-
Update on systemic therapies of metastatic renal cell carcinoma.World J Urol. 2010 Jun;28(3):303-9. doi: 10.1007/s00345-010-0519-5. Epub 2010 Feb 24. World J Urol. 2010. PMID: 20180125 Review.
Cited by
-
[Renal cell carcinoma: Drug therapy and prognostic models].Urologe A. 2015 May;54(5):735-46; quiz 747-8. doi: 10.1007/s00120-015-3845-9. Urologe A. 2015. PMID: 25987339 German.
-
Systematic Review: Perioperative Systemic Therapy for Metastatic Renal Cell Carcinoma.Kidney Cancer. 2017 Jul 26;1(1):57-64. doi: 10.3233/KCA-170009. Kidney Cancer. 2017. PMID: 30334005 Free PMC article.
-
Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma.Eur Urol. 2014 Nov;66(5):874-80. doi: 10.1016/j.eururo.2014.01.035. Epub 2014 Feb 7. Eur Urol. 2014. PMID: 24560330 Free PMC article. Clinical Trial.
-
Neoadjuvant Cabozantinib in an Unresectable Locally Advanced Renal Cell Carcinoma Patient Leads to Downsizing of Tumor Enabling Surgical Resection: A Case Report.Front Oncol. 2021 Feb 1;10:622134. doi: 10.3389/fonc.2020.622134. eCollection 2020. Front Oncol. 2021. PMID: 33598435 Free PMC article.
-
Management of advanced kidney cancer: Kidney Cancer Research Network of Canada (KCRNC) consensus update 2021.Can Urol Assoc J. 2021 Apr;15(4):84-97. doi: 10.5489/cuaj.7245. Can Urol Assoc J. 2021. PMID: 33830005 Free PMC article. No abstract available.
References
-
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. - PubMed
-
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. - PubMed
-
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. - PubMed
-
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

