Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate
- PMID: 20159823
- PMCID: PMC2849770
- DOI: 10.1200/JCO.2009.24.6819
Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate
Abstract
Purpose: The principal objective of this trial was to evaluate the antitumor activity of abiraterone acetate, an oral, specific, irreversible inhibitor of CYP17 in docetaxel-treated patients with castration-resistant prostate cancer (CRPC).
Patients and methods: In this multicenter, two-stage, phase II study, abiraterone acetate 1,000 mg was administered once daily continuously. The primary end point was achievement of a prostate-specific antigen (PSA) decline of > or = 50% in at least seven of 35 patients. Per an attained phase II design, more than 35 patients could be enrolled if the primary end point was met. Secondary objectives included: PSA declines of > or = 30% and > or = 90%; rate of RECIST (Response Evaluation Criteria in Solid Tumors) responses and duration on study; time to PSA progression; safety and tolerability; and circulating tumor cell (CTC) enumeration.
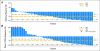
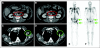
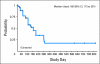
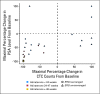
Results: Docetaxel-treated patients with CRPC (N = 47) were enrolled. PSA declines of > or = 30%, > or = 50% and > or = 90% were seen in 68% (32 of 47), 51% (24 of 47), and 15% (seven of 47) of patients, respectively. Partial responses (by RECIST) were reported in eight (27%) of 30 patients with measurable disease. Median time to PSA progression was 169 days (95% CI, 113 to 281 days). The median number of weeks on study was 24, and 12 (25.5%) of 47 patients remained on study > or = 48 weeks. CTCs were enumerated in 34 patients; 27 (79%) of 34 patients had at least five CTCs at baseline. Eleven (41%) of 27 patients had a decline from at least five to less than 5 CTCs, and 18 (67%) of 27 had a > or = 30% decline in CTCs after starting treatment with abiraterone acetate. Abiraterone acetate was well tolerated.
Conclusion: Abiraterone acetate has significant antitumor activity in post-docetaxel patients with CRPC. Randomized, phase III trials of abiraterone acetate are underway to define the future role of this agent.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Castration-resistant prostate cancer--hormone therapy redux.J Clin Oncol. 2010 Mar 20;28(9):1447-9. doi: 10.1200/JCO.2009.25.3781. Epub 2010 Feb 16. J Clin Oncol. 2010. PMID: 20159817 No abstract available.
-
Efficacy of abiraterone acetate in post-docetaxel castration-resistant prostate cancer.Expert Rev Anticancer Ther. 2010 Jul;10(7):1027-30. doi: 10.1586/era.10.84. Expert Rev Anticancer Ther. 2010. PMID: 20645691
References
-
- National Cancer Institute. Surveillance, Epidemiology and End Results. www.seer.cancer.gov.
-
- Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
-
- Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

