Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza
- PMID: 20159892
- PMCID: PMC2831695
- DOI: 10.1503/cmaj.092127
Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza
Abstract
Background: Whether the enteric absorption of the neuraminidase inhibitor oseltamivir is impaired in critically ill patients is unknown. We documented the pharmacokinetic profile of oseltamivir in patients admitted to intensive care units (ICUs) with suspected or confirmed pandemic (H1N1) influenza.
Methods: We included 41 patients 18 years of age and older with suspected or confirmed pandemic (H1N1) influenza who were admitted for ventilatory support to nine ICUs in three cities in Canada and Spain. Using tandem mass spectrometry, we assessed plasma levels of oseltamivir free base and its active metabolite carboxylate at baseline (before gastric administration of the drug) and at 2, 4, 6, 9 and 12 hours after the fourth or later dose.
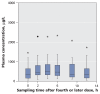
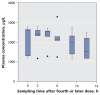
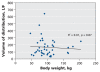
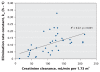
Results: Among the 36 patients who did not require dialysis, the median concentration of oseltamivir free base was 10.4 (interquartile range [IQR] 4.8-14.9) microg/L; the median concentration of the carboxylate metabolite was 404 (IQR 257-900) microg/L. The volume of distribution of the carboxylate metabolite did not increase with increasing body weight (R2=0.00, p=0.87). The rate of elimination of oseltamivir carboxylate was modestly correlated with estimations of creatinine clearance (R2=0.27, p<0.001). Drug clearance in the five patients who required continuous renal replacement therapy was about one-sixth that in the 36 patients with relatively normal renal function.
Interpretation: Oseltamivir was well absorbed enterically in critically ill patients admitted to the ICU with suspected or confirmed pandemic (H1N1) influenza. The dosage of 75 mg twice daily achieved plasma levels that were comparable to those in ambulatory patients and were far in excess of concentrations required to maximally inhibit neuraminidase activity of the virus. Adjustment of the dosage in patients with renal dysfunction requiring continuous renal replacement therapy is appropriate; adjustment for obesity does not appear to be necessary.
Figures
References
-
- Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. - PubMed
-
- Dominguez-Cherit G, Lapinsky SE, Espinosa-Perez L, et al. Critically ill patients with influenza A (novel H1N1) in Mexico. JAMA. 2009;302:1880–7. - PubMed
-
- World Health Organization. WHO guidelines for pharmacologic management of pandemic (H1N1) 2009 influenza and other influenza viruses. Geneva (Switzerland): The Organization; 2009.
-
- World Health Organization. Clinical management of human infection with pandemic (H1N1) 2009: revised guidance. Geneva (Switzerland): The Organization; 2009.
-
- Power BM, Forbes AM, van Heerden PV, et al. Pharmacokinetics of drugs used in critically ill adults. Clin Pharmacokinet. 1998;34:25–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
