Functional characterization of vasopressin type 2 receptor substitutions (R137H/C/L) leading to nephrogenic diabetes insipidus and nephrogenic syndrome of inappropriate antidiuresis: implications for treatments
- PMID: 20159941
- PMCID: PMC2872969
- DOI: 10.1124/mol.109.061804
Functional characterization of vasopressin type 2 receptor substitutions (R137H/C/L) leading to nephrogenic diabetes insipidus and nephrogenic syndrome of inappropriate antidiuresis: implications for treatments
Abstract
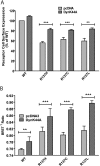
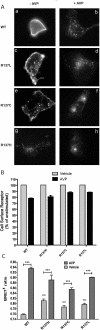
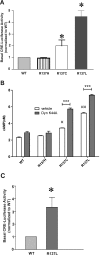
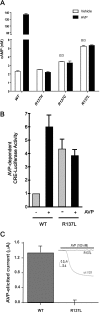
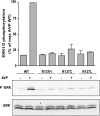
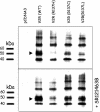
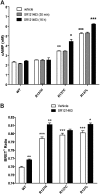
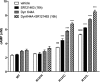
Substitution of arginine-137 of the vasopressin type 2 receptor (V2R) for histidine (R137H-V2R) leads to nephrogenic diabetes insipidus (NDI), whereas substitution of the same residue to cysteine or leucine (R137C/L-V2R) causes the nephrogenic syndrome of inappropriate antidiuresis (NSIAD). These two diseases have opposite clinical outcomes. Still, the three mutant receptors were shown to share constitutive beta-arrestin recruitment and endocytosis, resistance to vasopressin-stimulated cAMP production and mitogen-activated protein kinase activation, and compromised cell surface targeting, raising questions about the contribution of these phenomenons to the diseases and their potential treatments. Blocking endocytosis exacerbated the elevated basal cAMP levels promoted by R137C/L-V2R but not the cAMP production elicited by R137H-V2R, demonstrating that substitution of Arg137 to Cys/Leu, but not His, leads to constitutive V2R-stimulated cAMP accumulation that most likely underlies NSIAD. The constitutively elevated endocytosis of R137C/L-V2R attenuates the signaling and most likely reduces the severity of NSIAD, whereas the elevated endocytosis of R137H-V2R probably contributes to NDI. The constitutive signaling of R137C/L-V2R was not inhibited by treatment with the V2R inverse agonist satavaptan (SR121463). In contrast, owing to its pharmacological chaperone property, SR121463 increased the R137C/L-V2R maturation and cell surface targeting, leading to a further increase in basal cAMP production, thus disqualifying it as a potential treatment for patients with R137C/L-V2R-induced NSIAD. However, vasopressin was found to promote beta-arrestin/AP-2-dependent internalization of R137H/C/L-V2R beyond their already elevated endocytosis levels, raising the possibility that vasopressin could have a therapeutic value for patients with R137C/L-V2R-induced NSIAD by reducing steady-state surface receptor levels, thus lowering basal cAMP production.
Figures
Similar articles
-
Agonist-independent interactions between beta-arrestins and mutant vasopressin type II receptors associated with nephrogenic syndrome of inappropriate antidiuresis.Mol Endocrinol. 2009 Apr;23(4):559-71. doi: 10.1210/me.2008-0321. Epub 2009 Jan 29. Mol Endocrinol. 2009. PMID: 19179480 Free PMC article.
-
Characterization of three vasopressin receptor 2 variants: an apparent polymorphism (V266A) and two loss-of-function mutations (R181C and M311V).PLoS One. 2013 Jun 6;8(6):e65885. doi: 10.1371/journal.pone.0065885. Print 2013. PLoS One. 2013. PMID: 23762448 Free PMC article.
-
Mutation in the V2 vasopressin receptor gene, AVPR2, causes nephrogenic syndrome of inappropriate diuresis.Kidney Int. 2015 Nov;88(5):1070-8. doi: 10.1038/ki.2015.181. Epub 2015 Jul 1. Kidney Int. 2015. PMID: 26131744
-
V2 vasopressin receptor mutations.Vitam Horm. 2020;113:79-99. doi: 10.1016/bs.vh.2019.08.012. Epub 2019 Sep 13. Vitam Horm. 2020. PMID: 32138955 Review.
-
[Nephrogenic syndrome of inappropriate antidiuresis].Pan Afr Med J. 2019 Apr 29;32:210. doi: 10.11604/pamj.2019.32.210.6006. eCollection 2019. Pan Afr Med J. 2019. PMID: 31312322 Free PMC article. Review. French.
Cited by
-
Focus on neonatal and infantile onset of nephrogenic syndrome of inappropriate antidiuresis: 12 years later.Pediatr Nephrol. 2019 May;34(5):763-775. doi: 10.1007/s00467-018-3922-6. Epub 2018 Mar 15. Pediatr Nephrol. 2019. PMID: 29546600 Review.
-
Signaling Modification by GPCR Heteromer and Its Implication on X-Linked Nephrogenic Diabetes Insipidus.PLoS One. 2016 Sep 20;11(9):e0163086. doi: 10.1371/journal.pone.0163086. eCollection 2016. PLoS One. 2016. PMID: 27649563 Free PMC article.
-
Rare, functional, somatic variants in gene families linked to cancer genes: GPCR signaling as a paradigm.Oncogene. 2019 Sep;38(38):6491-6506. doi: 10.1038/s41388-019-0895-2. Epub 2019 Jul 23. Oncogene. 2019. PMID: 31337866 Free PMC article.
-
Biased signaling favoring gi over β-arrestin promoted by an apelin fragment lacking the C-terminal phenylalanine.J Biol Chem. 2014 Aug 29;289(35):24599-610. doi: 10.1074/jbc.M113.541698. Epub 2014 Jul 10. J Biol Chem. 2014. PMID: 25012663 Free PMC article.
-
Functional characterization of a loss-of-function mutant I324M of arginine vasopressin receptor 2 in X-linked nephrogenic diabetes insipidus.Sci Rep. 2021 May 26;11(1):11057. doi: 10.1038/s41598-021-90736-z. Sci Rep. 2021. PMID: 34040143 Free PMC article.
References
-
- Ball SG. (2007) Vasopressin and disorders of water balance: the physiology and pathophysiology of vasopressin. Ann Clin Biochem 44:417–431 - PubMed
-
- Barki-Harrington L, Rockman HA. (2008) β-Arrestins: multifunctional cellular mediators. Physiology (Bethesda) 23:17–22 - PubMed
-
- Bernier V, Bichet DG, Bouvier M. (2004a) Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol 4:528–533 - PubMed
-
- Bernier V, Lagacé M, Lonergan M, Arthus MF, Bichet DG, Bouvier M. (2004b) Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol Endocrinol 18:2074–2084 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

