GSK-3beta promotes cell survival by modulating Bif-1-dependent autophagy and cell death
- PMID: 20159967
- PMCID: PMC2831760
- DOI: 10.1242/jcs.060475
GSK-3beta promotes cell survival by modulating Bif-1-dependent autophagy and cell death
Abstract
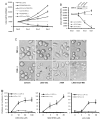
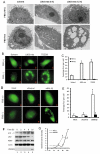
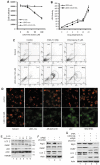
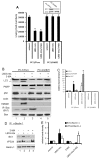
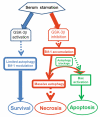
Glycogen synthase kinase 3 beta (GSK-3beta) is constantly active in cells and its activity increases after serum deprivation, indicating that GSK-3beta might play a major role in cell survival under serum starvation. In this study, we attempted to determine how GSK-3beta promotes cell survival after serum depletion. Under full culture conditions (10% FBS), GSK-3beta inhibition with chemical inhibitors or siRNAs failed to induce cell death in human prostate cancer cells. By contrast, under conditions of serum starvation, a profound necrotic cell death was observed as evidenced by cellular morphologic features and biochemical markers. Further analysis revealed that GSK-3beta-inhibition-induced cell death was in parallel with an extensive autophagic response. Interestingly, blocking the autophagic response switched GSK-3beta-inhibition-induced necrosis to apoptotic cell death. Finally, GSK-3beta inhibition resulted in a remarkable elevation of Bif-1 protein levels, and silencing Bif-1 expression abrogated GSK-3beta-inhibition-induced autophagic response and cell death. Taken together, our study suggests that GSK-3beta promotes cell survival by modulating Bif-1-dependent autophagic response and cell death.
Figures


Similar articles
-
GSK-3β controls autophagy by modulating LKB1-AMPK pathway in prostate cancer cells.Prostate. 2016 Feb;76(2):172-83. doi: 10.1002/pros.23106. Epub 2015 Oct 6. Prostate. 2016. PMID: 26440826 Free PMC article.
-
Regulation of autophagic cell death by glycogen synthase kinase-3β in adult hippocampal neural stem cells following insulin withdrawal.Mol Brain. 2015 May 19;8:30. doi: 10.1186/s13041-015-0119-9. Mol Brain. 2015. PMID: 25986948 Free PMC article.
-
Inhibition of glycogen synthase kinase-3 beta induces apoptosis and mitotic catastrophe by disrupting centrosome regulation in cancer cells.Sci Rep. 2015 Aug 21;5:13249. doi: 10.1038/srep13249. Sci Rep. 2015. PMID: 26292722 Free PMC article.
-
Glycogen synthase kinase-3beta suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer.Mol Cancer Ther. 2003 Nov;2(11):1215-22. Mol Cancer Ther. 2003. PMID: 14617795
-
Resveratrol protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death.Cell Death Dis. 2014 Mar 27;5(3):e1147. doi: 10.1038/cddis.2014.123. Cell Death Dis. 2014. PMID: 24675465 Free PMC article.
Cited by
-
Membrane microdomain-associated uroplakin IIIa contributes to Src-dependent mechanisms of anti-apoptotic proliferation in human bladder carcinoma cells.Biol Open. 2012 Oct 15;1(10):1024-34. doi: 10.1242/bio.20121115. Epub 2012 Aug 17. Biol Open. 2012. PMID: 23213380 Free PMC article.
-
Lithium: A Promising Anticancer Agent.Life (Basel). 2023 Feb 15;13(2):537. doi: 10.3390/life13020537. Life (Basel). 2023. PMID: 36836894 Free PMC article. Review.
-
High Levels of miR-7-5p Potentiate Crizotinib-Induced Cytokilling and Autophagic Flux by Targeting RAF1 in NPM-ALK Positive Lymphoma Cells.Cancers (Basel). 2020 Oct 13;12(10):2951. doi: 10.3390/cancers12102951. Cancers (Basel). 2020. PMID: 33066037 Free PMC article.
-
Molecular Mechanism of Autophagy: Its Role in the Therapy of Alzheimer's Disease.Curr Neuropharmacol. 2020;18(8):720-739. doi: 10.2174/1570159X18666200114163636. Curr Neuropharmacol. 2020. PMID: 31934838 Free PMC article. Review.
-
Blocking methionine catabolism induces senescence and confers vulnerability to GSK3 inhibition in liver cancer.Nat Cancer. 2024 Jan;5(1):131-146. doi: 10.1038/s43018-023-00671-3. Epub 2024 Jan 2. Nat Cancer. 2024. PMID: 38168934 Free PMC article.
References
-
- Bhat R., Xue Y., Berg S., Hellberg S., Ormö M., Nilsson Y., Radesäter A. C., Jerning E., Markgren P. O., Borgegård T., et al. (2003). Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J. Biol. Chem. 278, 45937-45945 - PubMed
-
- Boya P., Kroemer G. (2008). Lysosomal membrane permeabilization in cell death. Oncogene 27, 6434-6451 - PubMed
-
- Cao C., Subhawong T., Albert J. M., Kim K. W., Geng L., Sekhar K. R., Gi Y. J., Lu B. (2006). Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 66, 10040-10047 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

