Rapid modulation of the organic anion transporting polypeptide 2B1 (OATP2B1, SLCO2B1) function by protein kinase C-mediated internalization
- PMID: 20159975
- PMCID: PMC2857012
- DOI: 10.1074/jbc.M109.056457
Rapid modulation of the organic anion transporting polypeptide 2B1 (OATP2B1, SLCO2B1) function by protein kinase C-mediated internalization
Abstract
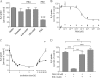
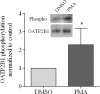
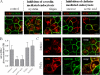
Members of the organic anion transporting polypeptide (OATP) family are involved in various pharmacological, pathophysiological, and physiological processes, such as hepatic drug uptake, progress of cancer, or transport of hormones. Although variability in expression and function of OATPs has been investigated in detail, data concerning regulation are rather limited. Here, we report a novel mechanism for rapid regulation of OATP2B1 mediated by protein kinase C (PKC) resulting in significant changes of transport activity. PKC activation by the phorbol ester (phorbol 12-myristate 13-acetate, PMA) resulted in increased phosphorylation of OATP2B1 as well as reduced OATP2B1 transport activity with a decrease in V(max) of E(1)S uptake (288 +/- 21 (control) versus 165 +/- 16 pmol/min/mg of protein (PMA)). This effect was sensitive to the PKC inhibitor bisindolylmaleimide I (BIM-I). Confocal microscopy, fluorescence-based internalization assay, and live-cell imaging using green fluorescent protein-tagged OATP2B1 revealed that transport inhibition was due to internalization of the transporter. Furthermore, colocalization with LAMP-2 and chloroquine-sensitive degradation of OATP2B1 suggest that the internalized protein is targeted to a lysosomal degradation pathway. With regard to the underlying mechanism inhibition of caveolin/lipid raft-mediated endocytosis failed to prevent OATP2B1 internalization, whereas inhibition of clathrin-mediated processes blocked OATP2B1 sequestration. However, small interfering RNA-mediated clathrin knock-down affected general trafficking of OATP2B1 and resulted in intracellular accumulation in the absence of PMA. In conclusion, our data demonstrate that OATP2B1 function is regulated by PKC-mediated, clathrin-dependent internalization and followed by lysosomal degradation. Furthermore, internalization could be shown in an ex vivo placenta perfusion. Our findings represent a new, rapid mechanism in regulation of human OATPs.
Figures





References
-
- Iwai M., Suzuki H., Ieiri I., Otsubo K., Sugiyama Y. (2004) Pharmacogenetics 14, 749–757 - PubMed
-
- Tirona R. G., Leake B. F., Merino G., Kim R. B. (2001) J. Biol. Chem. 276, 35669–35675 - PubMed
-
- Tachibana-Iimori R., Tabara Y., Kusuhara H., Kohara K., Kawamoto R., Nakura J., Tokunaga K., Kondo I., Sugiyama Y., Miki T. (2004) Drug Metab. Pharmacokinet. 19, 375–380 - PubMed
-
- Deng J. W., Song I. S., Shin H. J., Yeo C. W., Cho D. Y., Shon J. H., Shin J. G. (2008) Pharmacogenet. Genomics 18, 424–433 - PubMed
-
- Link E., Parish S., Armitage J., Bowman L., Heath S., Matsuda F., Gut I., Lathrop M., Collins R. (2008) N. Engl. J. Med. 359, 789–799 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

