Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils
- PMID: 20160017
- PMCID: PMC2863504
- DOI: 10.1128/IAI.01125-09
Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils
Abstract
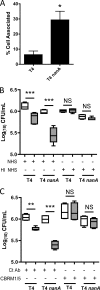
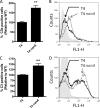
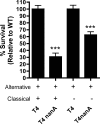
Streptococcus pneumoniae (the pneumococcus) is a major human pathogen and a leading cause of inflammatory infections such as pneumonia and otitis media. An important mechanism for host defense against S. pneumoniae is opsonophagocytic killing by neutrophils. To persist in the human host, the pneumococcus has developed strategies to evade opsonization and subsequent neutrophil-mediated killing. Utilizing a genomic approach, we identified NanA, the major pneumococcal neuraminidase, as a factor important for resistance to opsonophagocytic killing in ex vivo killing assays using human neutrophils. The effect of NanA was shown using both type 4 (TIGR4) and type 6A clinical isolates. NanA promotes this resistance by acting in conjunction with two other surface-associated exoglycosidases, BgaA, a beta-galactosidase, and StrH, an N-acetylglucosaminidase. Experiments using human serum showed that these exoglycosidases reduced deposition of complement component C3 on the pneumococcal surface, providing a mechanism for this resistance. Additionally, we have shown that antibodies in human serum do not contribute to this phenotype. These results demonstrate that deglycosylation of a human serum glycoconjugate(s) by the combined effects of NanA, BgaA, and StrH, is important for resistance to complement deposition and subsequent phagocytic killing of S. pneumoniae.
Figures
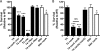
Similar articles
-
Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae.Mol Microbiol. 2006 Feb;59(3):961-74. doi: 10.1111/j.1365-2958.2005.04984.x. Mol Microbiol. 2006. PMID: 16420364
-
Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases.J Bacteriol. 2008 Jan;190(1):221-30. doi: 10.1128/JB.01251-07. Epub 2007 Nov 2. J Bacteriol. 2008. PMID: 17981977 Free PMC article.
-
Unravelling the multiple functions of the architecturally intricate Streptococcus pneumoniae β-galactosidase, BgaA.PLoS Pathog. 2014 Sep 11;10(9):e1004364. doi: 10.1371/journal.ppat.1004364. eCollection 2014 Sep. PLoS Pathog. 2014. PMID: 25210925 Free PMC article.
-
Role of Streptococcus pneumoniae extracellular glycosidases in immune evasion.Front Cell Infect Microbiol. 2023 Feb 3;13:1109449. doi: 10.3389/fcimb.2023.1109449. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36816580 Free PMC article. Review.
-
The Role of Neutrophils and Neutrophil Elastase in Pneumococcal Pneumonia.Front Cell Infect Microbiol. 2021 Mar 16;11:615959. doi: 10.3389/fcimb.2021.615959. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33796475 Free PMC article. Review.
Cited by
-
The Sialidase NanS Enhances Non-TcsL Mediated Cytotoxicity of Clostridium sordellii.Toxins (Basel). 2016 Jun 17;8(6):189. doi: 10.3390/toxins8060189. Toxins (Basel). 2016. PMID: 27322322 Free PMC article.
-
Molecular analysis of an enigmatic Streptococcus pneumoniae virulence factor: The raffinose-family oligosaccharide utilization system.J Biol Chem. 2019 Nov 15;294(46):17197-17208. doi: 10.1074/jbc.RA119.010280. Epub 2019 Oct 7. J Biol Chem. 2019. PMID: 31591266 Free PMC article.
-
Bacterial exploitation of phosphorylcholine mimicry suppresses inflammation to promote airway infection.J Clin Invest. 2015 Oct 1;125(10):3878-90. doi: 10.1172/JCI81888. Epub 2015 Aug 31. J Clin Invest. 2015. PMID: 26426079 Free PMC article.
-
Within-host microevolution of Streptococcus pneumoniae is rapid and adaptive during natural colonisation.Nat Commun. 2020 Jul 10;11(1):3442. doi: 10.1038/s41467-020-17327-w. Nat Commun. 2020. PMID: 32651390 Free PMC article.
-
NADH oxidase functions as an adhesin in Streptococcus pneumoniae and elicits a protective immune response in mice.PLoS One. 2013;8(4):e61128. doi: 10.1371/journal.pone.0061128. Epub 2013 Apr 8. PLoS One. 2013. PMID: 23577197 Free PMC article.
References
-
- Arnold, J. N., M. R. Wormald, R. B. Sim, P. M. Rudd, and R. A. Dwek. 2007. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 25:21-50. - PubMed
-
- Bolger, M. S., D. S. Ross, H. Jiang, M. M. Frank, A. J. Ghio, D. A. Schwartz, and J. R. Wright. 2007. Complement levels and activity in the normal and LPS-injured lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L748-L759. - PubMed
-
- Broides, A., E. Leibovitz, R. Dagan, J. Press, S. Raiz, M. Kafka, A. Leiberman, and T. Yermiahu. 2002. Cytology of middle ear fluid during acute otitis media. Pediatr. Infect. Dis. J. 21:57-61. - PubMed
-
- Brouwer, N., K. M. Dolman, M. van Houdt, M. Sta, D. Roos, and T. W. Kuijpers. 2008. Mannose-binding lectin (MBL) facilitates opsonophagocytosis of yeasts but not of bacteria despite MBL binding. J. Immunol. 180:4124-4132. - PubMed
-
- Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. U. S. A. 99:16969-16974. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

