Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance
- PMID: 20160039
- PMCID: PMC2836205
- DOI: 10.1158/0008-5472.CAN-09-2833
Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance
Abstract
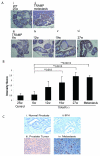
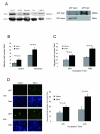
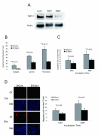
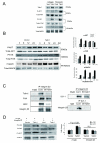
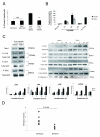
Talin1 is a focal adhesion complex protein that regulates integrin interactions with ECM. This study investigated the significance of talin1 in prostate cancer progression to metastasis in vitro and in vivo. Talin1 overexpression enhanced prostate cancer cell adhesion, migration, and invasion by activating survival signals and conferring resistance to anoikis. ShRNA-mediated talin1 loss led to a significant suppression of prostate cancer cell migration and transendothelial invasion in vitro and a significant inhibition of prostate cancer metastasis in vivo. Talin1-regulated cell survival signals via phosphorylation of focal adhesion complex proteins, such as focal adhesion kinase and Src, and downstream activation of AKT. Targeting AKT activation led to a significant reduction of talin1-mediated prostate cancer cell invasion. Furthermore, talin1 immunoreactivity directly correlated with prostate tumor progression to metastasis in the transgenic adenocarcinoma mouse prostate mouse model. Talin1 profiling in human prostate specimens revealed a significantly higher expression of cytoplasmic talin1 in metastatic tissue compared with primary prostate tumors (P < 0.0001). These findings suggest (a) a therapeutic significance of disrupting talin1 signaling/focal adhesion interactions in targeting metastatic prostate cancer and (b) a potential value for talin1 as a marker of tumor progression to metastasis.
Figures
References
-
- Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20(34):321–31. - PubMed
-
- Frisch SM, Screaton RA. Anoikis mechanisms. Current opinion in cell biology. 2001;13(5):555–62. - PubMed
-
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

