Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation
- PMID: 20160045
- PMCID: PMC2863620
- DOI: 10.1128/AAC.01529-09
Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation
Abstract
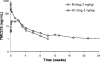
The next-generation human immunodeficiency virus type 1 (HIV-1) nonnucleoside reverse transcriptase inhibitor rilpivirine (TMC278) was administered in rats and dogs as single intramuscular (IM) or subcutaneous (SC) injections, formulated as a 200-nm nanosuspension. The plasma pharmacokinetics, injection site concentrations, disposition to lymphoid tissues, and tolerability were evaluated in support of its potential use as a once-monthly antiretroviral agent in humans. Rilpivirine plasma concentration-time profiles showed sustained and dose-proportional release over 2 months in rats and over 6 months in dogs. The absolute bioavailability approached 100%, indicating a complete release from the depot, in spite of rilpivirine concentrations still being high at the injection site(s) 3 months after administration in dogs. For both species, IM administration was associated with higher initial peak plasma concentrations and a more rapid washout than SC administration, which resulted in a stable plasma-concentration profile over at least 6 weeks in dogs. The rilpivirine concentrations in the lymph nodes draining the IM injection site exceeded the plasma concentrations by over 100-fold 1 month after administration, while the concentrations in the lymphoid tissues decreased to 3- to 6-fold the plasma concentrations beyond 3 months. These observations suggest uptake of nanoparticles by macrophages, which generates secondary depots in these lymph nodes. Both SC and IM injections were generally well tolerated and safe, with observations of a transient inflammatory response at the injection site. The findings support clinical investigations of rilpivirine nanosuspension as a long-acting formulation to improve adherence during antiretroviral therapy and for preexposure prophylaxis.
Figures

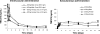

References
-
- Arasteh, K., A. Rieger, P. Yeni, A. Pozniak, G. Boogaerts, R. van Heeswijk, M. P. de Béthune, M. Peeters, and B. Woodfall. 2009. Short-term randomized proof-of-principle trial of TMC278 in patients with HIV type-1 who have previously failed antiretroviral therapy. Antivir. Ther. 14:713-722. - PubMed
-
- Baert, L., W. Dries, M. François, A. Wouters, E. Bastanie, K. Iterbeke, F. Stappers, P. Stevens, L. Schueller, G. van ′t Klooster, P. Van Remoortere, J. Rosier, G. Kraus, and P. Wigerinck. 2009. Development of a long-acting injectable formulation using nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur. J. Pharm. Biopharm. 72:502-508. - PubMed
-
- Bønnelykke Sørensen, V., H. Wroblewski, S. Galatius, S. Haunsø, and J. Kastrup. 2000. Assessment of continuous skeletal muscle blood flow during exercise in humans. Microvasc. Res. 59(2):301-309. - PubMed
-
- Burger, D. M., A. S. Bergshoeff, R. de Groot, D. Gibb, S. Walker, J.-M. Tréluye, and R. M. W. Hoetelmans, on behalf of the PENTA 5 Study Group. 2004. Maintaining the nelfinavir trough concentration above 0.8 mg/L improves virologic response in HIV-1-infected children. J. Pediatr. 145:403-405. - PubMed
-
- Connor, E. M., R. S. Sperling, R. Gelber, P. Kiselev, G. Scott, M. J. O'Sullivan, R. VanDyke, M. Bey, W. Shearer, R. L. Jacobson, E. Jimenez, E. O'Neill, B. Bazin, J.-F. Delfraissy, M. Culnane, R. Coombs, M. Elkins, J. Moye, P. Stratton, and J. Balsley. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N. Engl. J. Med. 331:1173-1180. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

