Single-dose pharmacokinetics of famciclovir in infants and population pharmacokinetic analysis in infants and children
- PMID: 20160046
- PMCID: PMC2863654
- DOI: 10.1128/AAC.01508-09
Single-dose pharmacokinetics of famciclovir in infants and population pharmacokinetic analysis in infants and children
Abstract
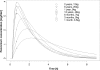
A multicenter, open-label study evaluated the single-dose pharmacokinetics and safety of a pediatric oral famciclovir (prodrug of penciclovir) formulation in infants aged 1 to 12 months with suspicion or evidence of herpes simplex virus infection. Individualized single doses of famciclovir based on the infant's body weight ranged from 25 to 175 mg. Eighteen infants were enrolled (1 to <3 months old [n = 8], 3 to <6 months old [n = 5], and 6 to 12 months old [n = 5]). Seventeen infants were included in the pharmacokinetic analysis; one infant experienced immediate emesis and was excluded. Mean C(max) and AUC(0-6) values of penciclovir in infants <6 months of age were approximately 3- to 4-fold lower than those in the 6- to 12-month age group. Specifically, mean AUC(0-6) was 2.2 microg h/ml in infants aged 1 to <3 months, 3.2 microg h/ml in infants aged 3 to <6 months, and 8.8 microg h/ml in infants aged 6 to 12 months. These data suggested that the dose administered to infants <6 months was less than optimal. Eight (44.4%) infants experienced at least one adverse event with gastrointestinal events reported most commonly. An updated pharmacokinetic analysis was conducted, which incorporated the data in infants from the present study and previously published data on children 1 to 12 years of age. An eight-step dosing regimen was derived that targeted exposure in infants and children 6 months to 12 years of age to match the penciclovir AUC seen in adults after a 500-mg dose of famciclovir.
Trial registration: ClinicalTrials.gov NCT00448227.
Figures





Similar articles
-
Pharmacokinetics and safety of famciclovir in children with herpes simplex or varicella-zoster virus infection.Antimicrob Agents Chemother. 2009 May;53(5):1912-20. doi: 10.1128/AAC.01054-08. Epub 2009 Mar 9. Antimicrob Agents Chemother. 2009. PMID: 19273678 Free PMC article. Clinical Trial.
-
Linear pharmacokinetics of penciclovir following administration of single oral doses of famciclovir 125, 250, 500 and 750 mg to healthy volunteers.J Antimicrob Chemother. 1994 Jan;33(1):119-27. doi: 10.1093/jac/33.1.119. J Antimicrob Chemother. 1994. PMID: 8157552 Clinical Trial.
-
Pharmacokinetics of penciclovir after oral administration of its prodrug famciclovir to horses.J Vet Med Sci. 2010 Mar;72(3):357-61. doi: 10.1292/jvms.09-0350. Epub 2009 Dec 4. J Vet Med Sci. 2010. PMID: 19959884
-
Pharmacology of new antiherpes agents: famciclovir and valacyclovir.J Am Pharm Assoc (Wash). 1997 Mar-Apr;NS37(2):157-63. doi: 10.1016/s1086-5802(16)30202-9. J Am Pharm Assoc (Wash). 1997. PMID: 9069689 Review.
-
Famciclovir. A review of its pharmacological properties and therapeutic efficacy in herpesvirus infections.Drugs. 1995 Aug;50(2):396-415. doi: 10.2165/00003495-199550020-00011. Drugs. 1995. PMID: 8521764 Review.
Cited by
-
Population pharmacokinetic analysis during the first 2 years of life: an overview.Clin Pharmacokinet. 2012 Dec;51(12):787-98. doi: 10.1007/s40262-012-0015-8. Clin Pharmacokinet. 2012. PMID: 23179579 Review.
-
Mitochondrial changes associated with viral infectious diseases in the paediatric population.Rev Med Virol. 2021 Nov;31(6):e2232. doi: 10.1002/rmv.2232. Epub 2021 Mar 31. Rev Med Virol. 2021. PMID: 33792105 Free PMC article.
-
Pharmacokinetic/pharmacodynamic modelling approaches in paediatric infectious diseases and immunology.Adv Drug Deliv Rev. 2014 Jun;73(100):127-39. doi: 10.1016/j.addr.2014.01.002. Epub 2014 Jan 17. Adv Drug Deliv Rev. 2014. PMID: 24440429 Free PMC article. Review.
References
-
- Abudalu, M., S. Tyring, W. Koltun, N. Bodsworth, and K. Hamed. 2008. Single-day, patient-initiated famciclovir therapy versus 3-day valacyclovir regimen for recurrent genital herpes: a randomized, double-blind, comparative trial. Clin. Infect. Dis. 47:651-658. - PubMed
-
- Anderson, B. J., and N. H. Holford. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303-332. - PubMed
-
- Aoki, F. Y., S. Tyring, F. Diaz-Mitoma, G. Gross, J. Gao, and K. Hamed. 2006. Single-day, patient-initiated famciclovir therapy for recurrent genital herpes: a randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 42:8-13. - PubMed
-
- Bodsworth, N., M. Bloch, A. McNulty, I. Denham, N. Doong, S. Trottier, M. Adena, M. A. Bonney, J. Agnew, et al. 2008. Two-day versus five-day famciclovir as treatment of recurrences of genital herpes: results of the FaST study. Sex. Health 5:219-225. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

