Characterization of UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase mutants of Proteus mirabilis: defectiveness in polymyxin B resistance, swarming, and virulence
- PMID: 20160049
- PMCID: PMC2863647
- DOI: 10.1128/AAC.01384-09
Characterization of UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase mutants of Proteus mirabilis: defectiveness in polymyxin B resistance, swarming, and virulence
Abstract
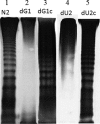
Proteus mirabilis is known to be highly resistant to the action of polymyxin B (PB). However, the mechanism underlying PB resistance is not clear. In this study, we used Tn5 transposon mutagenesis to identify genes that may affect PB resistance in P. mirabilis. Two genes, ugd and galU, which may encode UDP-glucose dehydrogenase (Ugd) and UDP-glucose pyrophosphorylase (GalU), respectively, were identified. Knockout mutants of ugd and galU were found to be extremely sensitive to PB, presumably because of alterations in lipopolysaccharide (LPS) structure and cell surface architecture in these mutants. These mutants were defective in swarming, expressed lower levels of virulence factor hemolysin, and had lower cell invasion ability. Complementation of the ugd or galU mutant with the full-length ugd or galU gene, respectively, led to the restoration of wild-type phenotypic traits. Interestingly, we found that the expression of Ugd and GalU was induced by PB through RppA, a putative response regulator of the bacterial two-component system that we identified previously. Mutation in either ugd or galU led to activation of RpoE, an extracytoplasmic function sigma factor that has been shown to be activated by protein misfolding and alterations in cell surface structure in other bacteria. Activation of RpoE or RpoE overexpression was found to cause inhibition of FlhDC and hemolysin expression. To our knowledge, this is the first report describing the roles and regulation of Ugd and GalU in P. mirabilis.
Figures


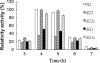
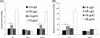
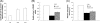
References
-
- Adams, P., R. Fowler, N. Kinsella, G. Howell, M. Farris, P. Coote, and C. D. O'Connor. 2001. Proteomic detection of PhoPQ- and acid-mediated repression of Salmonella motility. Proteomics 1:597-607. - PubMed
-
- Allison, C., H. C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 6:1583-1591. - PubMed
-
- Bader, M. W., W. W. Navarre, W. Shiau, H. Nikaido, J. G. Frye, M. McClelland, F. C. Fang, and S. I. Miller. 2003. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50:219-230. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

