Cycles, phase synchronization, and entrainment in single-species phytoplankton populations
- PMID: 20160096
- PMCID: PMC2840115
- DOI: 10.1073/pnas.0908725107
Cycles, phase synchronization, and entrainment in single-species phytoplankton populations
Abstract
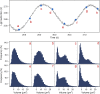
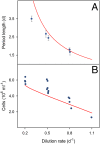
Complex dynamics, such as population cycles, can arise when the individual members of a population become synchronized. However, it is an open question how readily and through which mechanisms synchronization-driven cycles can occur in unstructured microbial populations. In experimental chemostats we studied large populations (>10(9) cells) of unicellular phytoplankton that displayed regular, inducible and reproducible population oscillations. Measurements of cell size distributions revealed that progression through the mitotic cycle was synchronized with the population cycles. A mathematical model that accounts for both the cell cycle and population-level processes suggests that cycles occur because individual cells become synchronized by interacting with one another through their common nutrient pool. An external perturbation by direct manipulation of the nutrient availability resulted in phase resetting, unmasking intrinsic oscillations and producing a transient collective cycle as the individuals gradually drift apart. Our study indicates a strong connection between complex within-cell processes and population dynamics, where synchronized cell cycles of unicellular phytoplankton provide sufficient population structure to cause small-amplitude oscillations at the population level.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Strogatz S. Sync: The Emerging Science of Spontaneous Order. New York: Hyperion; 2003.
-
- Pikovsky A, Rosenblum M, Kurths J. Synchronization—A Universal Concept in Nonlinear Sciences. Cambridge, UK: Cambridge Univ Press; 2001.
-
- Winfree AT. On emerging coherence. Science. 2001;298:2336–2337. - PubMed
-
- Taylor AF, Tinsley MR, Wang F, Huang Z, Showalter K. Dynamical quorum sensing and synchronization in large populations of chemical oscillators. Science. 2009;323:614–617. - PubMed
-
- Buck J. Synchronous rhythmic flashing of fireflies. Q Rev Biol. 1988;63:265–289. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

