Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival
- PMID: 20160097
- PMCID: PMC2840114
- DOI: 10.1073/pnas.0910261107
Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival
Abstract
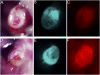
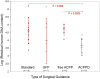
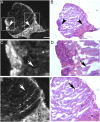
The completeness of tumor removal during surgery is dependent on the surgeon's ability to differentiate tumor from normal tissue using subjective criteria that are not easily quantifiable. A way to objectively assess tumor margins during surgery in patients would be of great value. We have developed a method to visualize tumors during surgery using activatable cell-penetrating peptides (ACPPs), in which the fluorescently labeled, polycationic cell-penetrating peptide (CPP) is coupled via a cleavable linker to a neutralizing peptide. Upon exposure to proteases characteristic of tumor tissue, the linker is cleaved, dissociating the inhibitory peptide and allowing the CPP to bind to and enter tumor cells. In mice, xenografts stably transfected with green fluorescent protein show colocalization with the Cy5-labeled ACPPs. In the same mouse models, Cy5-labeled free ACPPs and ACPPs conjugated to dendrimers (ACPPDs) delineate the margin between tumor and adjacent tissue, resulting in improved precision of tumor resection. Surgery guided by ACPPD resulted in fewer residual cancer cells left in the animal after surgery as measured by Alu PCR. A single injection of ACPPD dually labeled with Cy5 and gadolinium chelates enabled preoperative whole-body tumor detection by MRI, intraoperative guidance by real-time fluorescence, intraoperative histological analysis of margin status by fluorescence, and postoperative MRI tumor quantification. Animals whose tumors were resected with ACPPD guidance had better long-term tumor-free survival and overall survival than animals whose tumors were resected with traditional bright-field illumination only.
Conflict of interest statement
Conflict of interest statement: Q.T.N., E.S.O., T.A.A., T.J., and R.Y.T. have signed a scientific advisory agreement with a company founded to develop the technology described in this article.
Figures



References
-
- Ries LAG, et al. Bethesda, MD: National Cancer Institute; 2008. SEER Cancer Statistics Review, 1975–2005, http://seer.cancer.gov/csr/1975-2005/, based on November 2007 SEER data submission, posted to the SEER web site.
-
- Pattern of Care Studies/Quality of Care Studies . Bethesda, MD: National Cancer Institute,; 2008. SEER Cancer Statistics Review, 1975–2005. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site.
-
- Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–393. - PubMed
-
- Meric F, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer. 2003;97:926–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

