Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D(2) receptors
- PMID: 20160122
- PMCID: PMC2840813
- DOI: 10.1210/me.2009-0369
Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D(2) receptors
Abstract
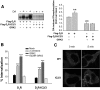
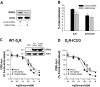
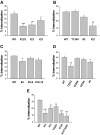
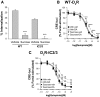
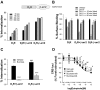
The regulatory mechanisms and functional roles of agonist-induced internalization of G protein-coupled receptors (GPCRs) were analyzed using mutant dopamine D(2) receptors (D(2)Rs) in which all possible GPCR kinase (GRK) phosphorylation sites were mutated or the affinity for beta-arrestins was altered. Agonist-induced internalization of D(2)Rs involved a phosphorylation-dependent component, which was mediated by serine/threonine (S/T) residues in the second loop and T225 in the third loop, and a phosphorylation-independent component. GRK2-mediated enhancement of the internalization and inhibition of D(2)R signaling did not involve receptor phosphorylation, and only the former required the enzymatic activity of GRK2. The phosphorylation-deficient mutant (D(2)R-intracellular loop 2/3) recycled more slowly and showed more agonist-induced desensitization than did the wild-type D(2)R, suggesting that receptor phosphorylation mediates the recycling of the internalized receptors and enhances receptor resensitization. Blockade of the agonist-induced internalization of D(2)R-intracellular loop 2/3 provoked desensitization as in wild-type D(2)R, suggesting that certain cellular processes other than receptor dephosphorylation occurring within the endocytic vesicle are responsible for the resensitization of D(2)R. When dissociation between D(2)R and beta-arrestin was inhibited or when the expression of cellular beta-arrestins was decreased, agonist-induced desensitization of D(2)R did not occur, suggesting that dissociation from beta-arrestin is the main cellular process required for resensitization of D(2)R and is achieved through agonist-induced internalization. These results indicate that, in the regulation of some GPCRs, phosphorylation-independent association with beta-arrestin plays a major role in agonist-induced desensitization.
Figures
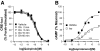

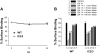

Similar articles
-
G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor.J Biol Chem. 2009 May 29;284(22):15038-51. doi: 10.1074/jbc.M900388200. Epub 2009 Mar 30. J Biol Chem. 2009. PMID: 19332542 Free PMC article.
-
Novel roles for β-arrestins in the regulation of pharmacological sequestration to predict agonist-induced desensitization of dopamine D3 receptors.Br J Pharmacol. 2013 Nov;170(5):1112-29. doi: 10.1111/bph.12357. Br J Pharmacol. 2013. PMID: 23992580 Free PMC article.
-
ARF6 and GASP-1 are post-endocytic sorting proteins selectively involved in the intracellular trafficking of dopamine D₂ receptors mediated by GRK and PKC in transfected cells.Br J Pharmacol. 2013 Mar;168(6):1355-74. doi: 10.1111/bph.12025. Br J Pharmacol. 2013. PMID: 23082996 Free PMC article.
-
Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling.Pharmacol Rev. 2001 Mar;53(1):1-24. Pharmacol Rev. 2001. PMID: 11171937 Review.
-
Molecular mechanisms of G protein-coupled receptor desensitization and resensitization.Life Sci. 1998;62(17-18):1561-5. doi: 10.1016/s0024-3205(98)00107-6. Life Sci. 1998. PMID: 9585136 Review.
Cited by
-
Dopamine and renal function and blood pressure regulation.Compr Physiol. 2011 Jul;1(3):1075-117. doi: 10.1002/cphy.c100032. Compr Physiol. 2011. PMID: 23733636 Free PMC article. Review.
-
Molecular Signature That Determines the Acute Tolerance of G Protein-Coupled Receptors.Biomol Ther (Seoul). 2017 May 1;25(3):239-248. doi: 10.4062/biomolther.2016.193. Biomol Ther (Seoul). 2017. PMID: 27956717 Free PMC article.
-
Anaplastic Lymphoma Kinase Regulates Internalization of the Dopamine D2 Receptor.Mol Pharmacol. 2020 Feb;97(2):123-131. doi: 10.1124/mol.119.117473. Epub 2019 Nov 16. Mol Pharmacol. 2020. PMID: 31734646 Free PMC article.
-
TRAF6-mediated ubiquitination of AKT in the nucleus is a critical event underlying the desensitization of G protein-coupled receptors.Cell Commun Signal. 2024 Apr 2;22(1):213. doi: 10.1186/s12964-024-01592-z. Cell Commun Signal. 2024. PMID: 38566235 Free PMC article.
-
Elucidation of G-protein and β-arrestin functional selectivity at the dopamine D2 receptor.Proc Natl Acad Sci U S A. 2015 Jun 2;112(22):7097-102. doi: 10.1073/pnas.1502742112. Epub 2015 May 11. Proc Natl Acad Sci U S A. 2015. PMID: 25964346 Free PMC article.
References
-
- Hausdorff WP, Caron MG, Lefkowitz RJ 1990 Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J 4:2881–2889 - PubMed
-
- Gurevich VV, Benovic JL 1997 Mechanism of phosphorylation-recognition by visual arrestin and the transition of arrestin into a high affinity binding state. Mol Pharmacol 51:161–169 - PubMed
-
- Ferguson SS, Downey III WE, Colapietro AM, Barak LS, Ménard L, Caron MG 1996 Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271:363–366 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

