Human cytochrome p450c17: single step purification and phosphorylation of serine 258 by protein kinase a
- PMID: 20160131
- PMCID: PMC2850244
- DOI: 10.1210/en.2009-1247
Human cytochrome p450c17: single step purification and phosphorylation of serine 258 by protein kinase a
Abstract
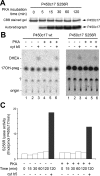
Cytochrome P450c17 (P450c17) is the single microsomal enzyme that catalyzes steroid 17alpha-hydroxylase and 17,20 lyase activities. The ratio of lyase to hydroxylase activity of human P450c17 determines whether steroidogenesis leads to the synthesis of cortisol or sex steroids. This ratio is regulated posttranslationally by factors that influence the efficiency of electron transfer from P450 oxidoreductase to P450c17. One factor favoring more efficient electron transfer and 17,20 lyase activity is cAMP-dependent serine/threonine phosphorylation of P450c17. Identifying the responsible kinase(s) and the P450c17 residues that undergo phosphorylation has been challenging, partly because of difficulties in preparing biochemically useful amounts of pure, catalytically active P450c17. We describe a modified strategy for preparing P450c17 in which the traditional carboxy-terminal 4xHis tag is replaced by 3xGly6xHis. This construct permits more rotational freedom of the protein when bound to the nickel affinity column, reducing steric associations between the protein and the column, and permitting a single-step chromatographic purification to apparent homogeneity. Using this vector, we explored P450c17 phosphorylation by mutagenesis of Ser and/or Thr residues to Asp or Glu to mimic the approximate size and charge of phospho-Ser or phospho-Thr. This strategy did not identify Ser and/or Thr site(s) that increase the ratio of lyase to hydroxylase activity, suggesting that the regulatory phosphorylation strategy of human P450c17 is very complicated. Although previous work has excluded protein kinase A (PKA) as the responsible kinase, the cAMP-inducible nature of the phosphorylation-associated increase in lyase activity suggests that PKA may play a role, possibly as a priming kinase. Using our novel vector and a series of mutations, we identified the P450c17 site phosphorylated by PKA as Ser258.
Figures

Similar articles
-
The regulation of 17,20 lyase activity.Steroids. 1997 Jan;62(1):133-42. doi: 10.1016/s0039-128x(96)00172-9. Steroids. 1997. PMID: 9029728 Review.
-
Steroid 17 alpha-hydroxylase and 17,20-lyase activities of P450c17: contributions of serine106 and P450 reductase.Endocrinology. 1993 Jun;132(6):2498-506. doi: 10.1210/endo.132.6.8504753. Endocrinology. 1993. PMID: 8504753
-
Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17.J Biol Chem. 2005 Apr 8;280(14):13265-71. doi: 10.1074/jbc.M414673200. Epub 2005 Feb 1. J Biol Chem. 2005. PMID: 15687493
-
Pathways leading to phosphorylation of p450c17 and to the posttranslational regulation of androgen biosynthesis.Endocrinology. 2008 May;149(5):2667-77. doi: 10.1210/en.2007-1527. Epub 2008 Jan 10. Endocrinology. 2008. PMID: 18187541 Free PMC article.
-
The post-translational regulation of 17,20 lyase activity.Mol Cell Endocrinol. 2015 Jun 15;408:99-106. doi: 10.1016/j.mce.2014.09.010. Epub 2014 Sep 16. Mol Cell Endocrinol. 2015. PMID: 25224484 Review.
Cited by
-
Cytochrome b5 Activates the 17,20-Lyase Activity of Human Cytochrome P450 17A1 by Increasing the Coupling of NADPH Consumption to Androgen Production.Biochemistry. 2016 Aug 9;55(31):4356-65. doi: 10.1021/acs.biochem.6b00532. Epub 2016 Jul 29. Biochemistry. 2016. PMID: 27426448 Free PMC article.
-
Minor activities and transition state properties of the human steroid hydroxylases cytochromes P450c17 and P450c21, from reactions observed with deuterium-labeled substrates.Biochemistry. 2012 Sep 11;51(36):7064-77. doi: 10.1021/bi300895w. Epub 2012 Aug 27. Biochemistry. 2012. PMID: 22873692 Free PMC article.
-
Effect of Essential Oil Components on the Activity of Steroidogenic Cytochrome P450.Biomolecules. 2024 Feb 8;14(2):203. doi: 10.3390/biom14020203. Biomolecules. 2024. PMID: 38397440 Free PMC article.
-
Tight binding of cytochrome b5 to cytochrome P450 17A1 is a critical feature of stimulation of C21 steroid lyase activity and androgen synthesis.J Biol Chem. 2021 Jan-Jun;296:100571. doi: 10.1016/j.jbc.2021.100571. Epub 2021 Mar 20. J Biol Chem. 2021. PMID: 33753170 Free PMC article.
-
Catalytic modulation of human cytochromes P450 17A1 and P450 11B2 by phospholipid.J Steroid Biochem Mol Biol. 2018 Jul;181:63-72. doi: 10.1016/j.jsbmb.2018.03.003. Epub 2018 Mar 13. J Steroid Biochem Mol Biol. 2018. PMID: 29548669 Free PMC article.
References
-
- Nakajin S, Shively JE, Yuan PM, Hall PF 1981 Microsomal cytochrome P450 from neonatal pig testis: two enzymatic activities (17α-hydroxylase and C17,20-lyase) associated with one protein. Biochemistry 20:4037–4042 - PubMed
-
- Nakajin S, Hall PF 1981 Microsomal cytochrome P450 from neonatal pig testis. Purification and properties of a C21 steroid side-chain cleavage system (17α-hydroxylase-C17,20 lyase). J Biol Chem 256:3871–3876 - PubMed
-
- Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF 1984 C21 steroid side-chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17α-hydroxylase/C17,20 lyase cytochrome P450. J Biol Chem 259:3971–3976 - PubMed
-
- Picado-Leonard J, Miller WL 1987 Cloning and sequence of the human gene encoding P450c17 (steroid 17α-hydroxylase/17,20 lyase): similarity to the gene for P450c21. DNA 6:439–448 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

