Proteasomal regulation of pulmonary fibrosis
- PMID: 20160152
- PMCID: PMC3137153
- DOI: 10.1513/pats.200906-055JS
Proteasomal regulation of pulmonary fibrosis
Abstract
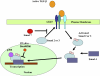
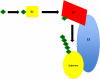
It is estimated that, combined, 400,000 people are diagnosed with idiopathic pulmonary fibrosis (IPF) or acute lung injury/acute respiratory distress syndrome annually in the United States, and both diseases are associated with an unacceptably high mortality rate. Although these disorders are distinct clinical entities, they share pathogenic mechanisms that may provide overlapping therapeutic targets. One example is fibroblast activation, which occurs concomitant with acute lung injury as well as in the progressive fibrosis of IPF. Both clinical entities are characterized by elevations of the profibrotic cytokine, transforming growth factor (TGF)-beta1. Protein degradation by the ubiquitin-proteasomal system modulates TGF-beta1 expression and signaling. In this review, we highlight the effects of proteasomal inhibition in various animal models of tissue fibrosis and mechanisms by which it may regulate TGF-beta1 expression and signaling. At present, there are no effective therapies for fibroproliferative acute respiratory distress syndrome or IPF, and proteasomal inhibition may provide a novel, attractive target in these devastating diseases.
Figures
Similar articles
-
Proteasomal inhibition after injury prevents fibrosis by modulating TGF-β(1) signalling.Thorax. 2012 Feb;67(2):139-46. doi: 10.1136/thoraxjnl-2011-200717. Epub 2011 Sep 15. Thorax. 2012. PMID: 21921091 Free PMC article.
-
2,4,6-trinitrobenzene sulfonic acid-induced chronic colitis with fibrosis and modulation of TGF-β1 signaling.World J Gastroenterol. 2014 Dec 28;20(48):18207-15. doi: 10.3748/wjg.v20.i48.18207. World J Gastroenterol. 2014. PMID: 25561788 Free PMC article.
-
Ubiquitin and cancer: from molecular targets and mechanisms to the clinic -- AACR Special Conference.IDrugs. 2006 Mar;9(3):179-81. IDrugs. 2006. PMID: 16523381 No abstract available.
-
The ubiquitin-proteasome system (UPS) and the mechanism of action of bortezomib.Curr Pharm Des. 2011;17(15):1483-99. doi: 10.2174/138161211796197124. Curr Pharm Des. 2011. PMID: 21504411 Review.
-
Proteasome inhibition as a therapeutic strategy for hematologic malignancies.Expert Rev Anticancer Ther. 2005 Jun;5(3):465-76. doi: 10.1586/14737140.5.3.465. Expert Rev Anticancer Ther. 2005. PMID: 16001954 Review.
Cited by
-
Tenascin-C deficiency attenuates TGF-ß-mediated fibrosis following murine lung injury.Am J Physiol Lung Cell Mol Physiol. 2010 Dec;299(6):L785-93. doi: 10.1152/ajplung.00385.2009. Epub 2010 Sep 10. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20833777 Free PMC article.
-
Pneumocyte injury and ubiquitin-positive pneumocytes in interstitial lung diseases.Histopathology. 2015 Jan;66(2):161-72. doi: 10.1111/his.12528. Epub 2014 Oct 30. Histopathology. 2015. PMID: 25123224 Free PMC article. Review.
-
Validation of the 2nd Generation Proteasome Inhibitor Oprozomib for Local Therapy of Pulmonary Fibrosis.PLoS One. 2015 Sep 4;10(9):e0136188. doi: 10.1371/journal.pone.0136188. eCollection 2015. PLoS One. 2015. PMID: 26340365 Free PMC article.
-
Bortezomib-induced bronchiolitis obliterans organizing pneumonia.Case Rep Pulmonol. 2012;2012:430141. doi: 10.1155/2012/430141. Epub 2012 Oct 21. Case Rep Pulmonol. 2012. PMID: 23133779 Free PMC article.
-
Human embryonic lung fibroblasts treated with artesunate exhibit reduced rates of proliferation and human cytomegalovirus infection in vitro.J Thorac Dis. 2015 Jul;7(7):1151-7. doi: 10.3978/j.issn.2072-1439.2015.07.05. J Thorac Dis. 2015. PMID: 26380730 Free PMC article.
References
-
- Martin C, Papazian L, Payan MJ, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome: a study in mechanically ventilated patients. Chest 1995;107:196–200. (see comments). - PubMed
-
- Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol 2005;175:5390–5395. - PubMed
-
- Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med 2000;21:435–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
